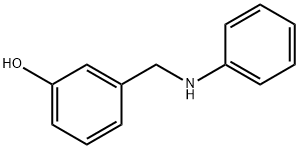
CHEMBRDG-BB 9071633 synthesis
- Product Name:CHEMBRDG-BB 9071633
- CAS Number:93189-07-2
- Molecular formula:C13H13NO
- Molecular Weight:199.25

100-83-4
611 suppliers
$5.00/10g

62-53-3
619 suppliers
$10.00/1g

93189-07-2
9 suppliers
$60.00/100mg
Yield:93189-07-2 100%
Reaction Conditions:
Stage #1: meta-hydroxybenzaldehyde;anilinewith acetic acid in dichloromethane at 20; for 24 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane at 20; for 24 h;
Steps:
1 Step 1: Preparation of 3-((phenylamino)methyl)phenol (I-62a)
To a solution of 3-hydroxybenzaldehyde (1.05 g, 8.6 mmol) and aniline (821 jiL,9.0 mmol) in dichloromethane (35 mL) was added acetic acid (516 jiL, 9.0 mmol). The reaction mixture was stirred at room temperature for 24 h. Sodium triacetoxyborohydride (4.55 g, 21.5 mmol) was added and the reaction was stirred at room temperature for 24 h. Water was added to quench the reaction and the mixture was diluted with dichloromethane. The organic layer was washed with brine, passed through ahydrophobic frit and the solvent was removed in vacuo to afford the title compound as a brown oil (1.7 g, quantitative yield).‘H NMR (400 MHz, CDC13): ? 7.25-7.15 (m, 3 H), 6.94-6.92 (m, 1 H), 6.85 (s, 1 H), 6.74-6.69 (m, 2 H), 6.63-6.60 (m, 2 H), 4.71 (brs, 1 H), 4.29 (s, 2 H), 4.11 (brs, 1 H). LCMS (Method 1): [MH+] = 200 at 3.48 mm.
References:
WO2014/86852,2014,A1 Location in patent:Page/Page column 70; 71
![Phenol, 3-[(phenylimino)methyl]-](/CAS/20180601/GIF/13206-60-5.gif)
13206-60-5
0 suppliers
inquiry

93189-07-2
9 suppliers
$60.00/100mg
27559-45-1
25 suppliers
$65.00/50mg

93189-07-2
9 suppliers
$60.00/100mg

100-83-4
611 suppliers
$5.00/10g

93189-07-2
9 suppliers
$60.00/100mg

62-53-3
619 suppliers
$10.00/1g

93189-07-2
9 suppliers
$60.00/100mg