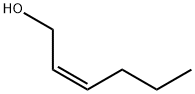
CIS-2-HEXEN-1-OL synthesis
- Product Name:CIS-2-HEXEN-1-OL
- CAS Number:928-94-9
- Molecular formula:C6H12O
- Molecular Weight:100.16

505-57-7
17 suppliers
inquiry

928-94-9
96 suppliers
$36.79/5g
Yield:928-94-9 > 99 %Chromat.
Reaction Conditions:
with sodium tetrahydroborate;erbium(III) triflate in 2-methyltetrahydrofuran at 20; for 0.0833333 h;Green chemistry;regioselective reaction;
Steps:
14 4.2 General procedure for the stereoselective reduction of α,β-unsaturated carbonyl compounds
General procedure: To a suspension of α,β-unsaturated carbonyl compound (2.0 mmol) and Er(OTf)3 (0.1 mmol) in 2-MeTHF (3 mL) an equimolar quantity of NaBH4 (2.0 mmol) was added. The reaction mixture was stirred at room temperature and monitored by GC/MS until consumption of starting material. The crude reaction mixture was quenched with H2O (3 mL), the organic phase was dried on dry Na2SO4 and the solvent was evaporated under reduced pressure. The desired product was obtained pure after work-up.
References:
Nardi, Monica;Sindona, Giovanni;Costanzo, Paola;Oliverio, Manuela;Procopio, Antonio [Tetrahedron,2015,vol. 71,# 7,p. 1132 - 1135]

764-60-3
105 suppliers
$53.29/5g

928-94-9
96 suppliers
$36.79/5g
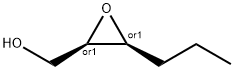
90528-63-5
0 suppliers
inquiry

928-94-9
96 suppliers
$36.79/5g
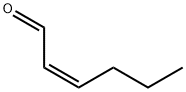
16635-54-4
10 suppliers
inquiry

928-94-9
96 suppliers
$36.79/5g
