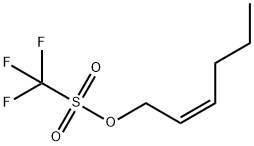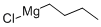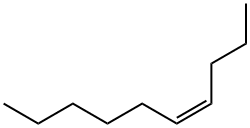
CIS-4-DECENE synthesis
- Product Name:CIS-4-DECENE
- CAS Number:19398-88-0
- Molecular formula:C10H20
- Molecular Weight:140.27

2384-86-3
50 suppliers
$42.00/5mL

19398-88-0
9 suppliers
inquiry
Yield:19398-88-0 100 %Chromat.
Reaction Conditions:
with potassium tert-butylate;copper(II) acetate monohydrate;1,3-bis[2,6-diisopropylphenyl]imidazolium chloride;tert-butyl alcohol in toluene at 50; for 20 h;Catalytic behavior;Inert atmosphere;diastereoselective reaction;Reagent/catalyst;Solvent;
Steps:
General procedure for the copper-catalyzed semihydrogenation of alkynes: for liquid substrates (1a, b, c, e, k, o, p, q, t, u, v, x, y, aa, ab, ac)
General procedure: In air, Cu(OAc)2·H2O (5.0mg, 5mol%) and IPr·HCl (10.6mg, 5mol%) were placed in a screw-capped reaction vial. The vial was moved in to a glove box and t-BuOK (5.6mg, 10mol%) and solvent (1.0ml) were added. The vial was moved out of the glove box and connected to an argon line through a needle. The mixture was raised to 50°C and stirred for 1h. PMHS (131mg, 4.0equiv) wasthen added dropwise with a microsyringe and the solution was stirred for an additional 30min. After the mixture was changed to the specified reaction temperature, liquid alkyne (0.5mmol) and t-BuOH (74mg, 2.0equiv) was added dropwise. The mixture was stirred for a specified period of time. The reaction mixture was subsequently hydrolyzed by adding 1M NaOH aqueous (2ml) (for substrates with no hydroxyl group) or 1M TBAF in THF (2ml) at 0°C (for substrates with a hydroxyl group) for several hours. The mixture was extracted with ether (2ml×3). Crude products wereobtained after evaporation and purified by silica gel chromatography.
References:
Wang, Guang-Hui;Bin, Huai-Yu;Sun, Miao;Chen, Shu-Wei;Liu, Ji-Hong;Zhong, Chong-Min [Tetrahedron,2014,vol. 70,# 12,p. 2175 - 2179] Location in patent:supporting information

2384-86-3
50 suppliers
$42.00/5mL
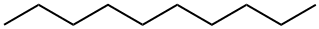
124-18-5
258 suppliers
$24.50/1000mg

19398-88-0
9 suppliers
inquiry
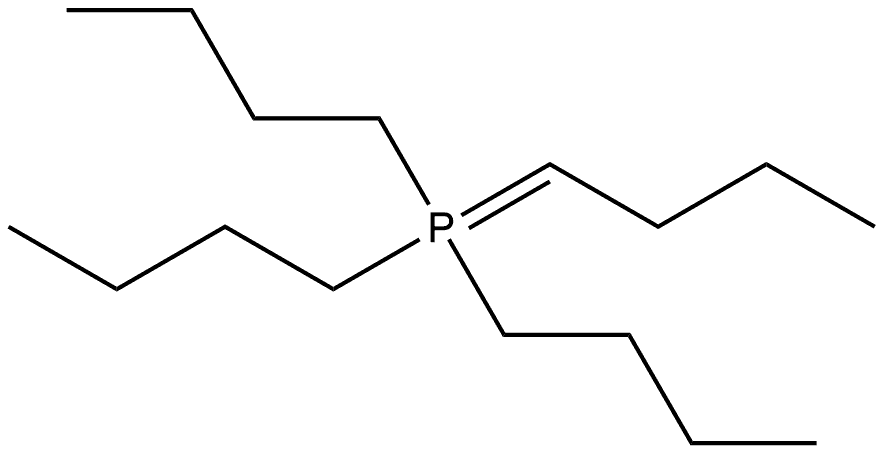
43216-19-9
0 suppliers
inquiry

66-25-1
335 suppliers
$16.80/100G

19398-88-0
9 suppliers
inquiry
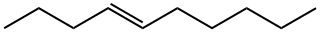
19398-89-1
18 suppliers
inquiry