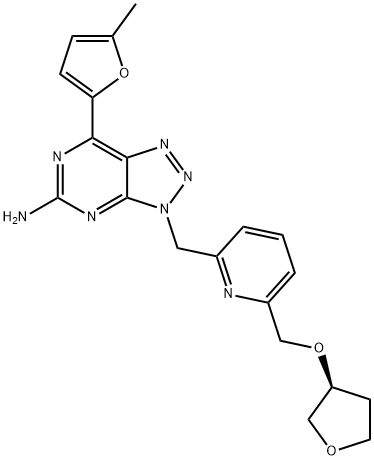
CPI-444 synthesis
- Product Name:CPI-444
- CAS Number:1202402-40-1
- Molecular formula:C20H21N7O3
- Molecular Weight:407.43
![(S)-7-chloro-3-((6-(((tetrahydrofuran-3-yl)oxy)methyl)pyridin-2-yl)methyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-amine](/CAS/20200119/GIF/1202402-82-1.gif)
1202402-82-1
1 suppliers
inquiry
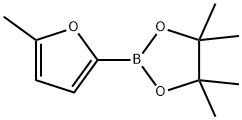
338998-93-9
110 suppliers
$6.00/1g

1202402-40-1
96 suppliers
$39.00/1mg
Yield:1202402-40-1 90%
Reaction Conditions:
with dichloro[1,1′-bis[bis(1,1-dimethylethyl)phosphino]ferrocene-P,P′]palladium;potassium carbonate in tetrahydrofuran;water at 60;Suzuki Coupling;Temperature;
Steps:
4
The solution of CP-58 (10 g), CP-60 (6.9 g) , Pd(dtbpf)C12 (approx. 0.0015 mol eq) and K2C03 (5.8 g) in THF (6V) and H20 (3V) is heated to approximately 60 °C. The reaction is complete after approximately 30 minutes. The solution is cooled to 50 °C and aqueous layer is separated. The aqueous layer is extracted with THF (9 mL); the THF layer is added to organic solution. The organics are cooled to 40 °C, 1 ,3-diaminopropane (DAP; approximately 50 g) or ethylene diamine (EDA; approximately 45 g) is added and the mixture stined for 1 hour. H20 (15V) is added to the organic layer over 10 mm. The sluny is cooled to 20 °C for 2 hours, and stined for an additional 1 hour. The sluny is filtered and washed with H20 (2V x 2) and iPrOH (lv). CPI-444 wet-cake is dried at 50 °C under full vacuum. (Yield = 90 %; purity> 99.0%)
References:
WO2018/183965,2018,A1 Location in patent:Paragraph 0350; 0351; 0352; 0353; 0354

338998-93-9
110 suppliers
$6.00/1g

1202402-40-1
96 suppliers
$39.00/1mg
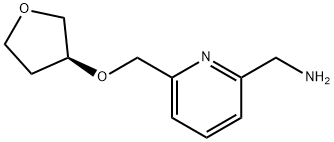
1202402-79-6
18 suppliers
inquiry

1202402-40-1
96 suppliers
$39.00/1mg