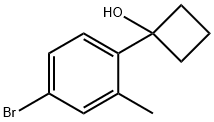
Cyclobutanol, 1-(4-bromo-2-methylphenyl)- synthesis
- Product Name:Cyclobutanol, 1-(4-bromo-2-methylphenyl)-
- CAS Number:1467060-73-6
- Molecular formula:C11H13BrO
- Molecular Weight:241.12
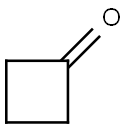
1191-95-3
329 suppliers
$10.00/1g
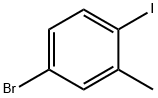
116632-39-4
206 suppliers
$8.00/5g

1467060-73-6
6 suppliers
inquiry
Yield:1467060-73-6 73%
Reaction Conditions:
Stage #1: 4-bromo-1-iodo-2-methylbenzenewith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.75 h;
Stage #2: cyclobutanone in tetrahydrofuran;hexane at -78; for 1.5 h;
Stage #3: with lithium hydroxide monohydrate;ammonia hydrochloride in tetrahydrofuran;lithium hydroxide monohydrate;
Steps:
41.1 1-(4-bromo-2-methylphenyl)cyclobutanol
To a solution of 4-bromo-1-iodo-2-methylbenzene (0.24 mL, 1.6 mmol) in tetrahydrofuran (5 mL) at -78° C. was added n-butyl lithium (2.5 M in hexane, 0.8 mL, 2.0 mmol) dropwise over 15 minutes.
The reaction mixture was stirred at -78° C. for 30 minutes, treated with neat cyclobutanone (0.12 mL, 1.6 mmol) dropwise over 10 minutes and stirred at -78° C. for an additional 1.5 hours.
The reaction was quenched with saturated aqueous ammonium chloride solution, warmed to room temperature and extracted with ethyl acetate (2*).
The combined organic layers were dried over magnesium sulfate, filtered and concentrated in vacuo to afford 500 mg yellow semi-solid, which was purified by flash chromatography (12 g silica, 0-50% ethyl acetate/heptane, 18 column volumes).
Product fractions were combined and concentrated in vacuo to afford the title compound as a colorless waxy solid (290 mg, 73% yield). GCMS 240/242 (M)+; 1H NMR (400 MHz, CDCl3) δ 7.23-7.31 (m, 2H), 7.11 (d, J=8.20 Hz, 1H), 2.55-2.64 (m, 2H), 2.28-2.38 (m, 5H), 2.07-2.20 (m, 1H), 1.93 (s, 1H), 1.62-1.74 (m, 1H).
References:
US2013/267493,2013,A1 Location in patent:Paragraph 0910