
Cyclobutanone, 2-(2,4-dichlorophenyl)- synthesis
- Product Name:Cyclobutanone, 2-(2,4-dichlorophenyl)-
- CAS Number:1261038-31-6
- Molecular formula:C10H8Cl2O
- Molecular Weight:215.08

1134916-54-3
1 suppliers
inquiry

1261038-31-6
9 suppliers
inquiry
Yield:1261038-31-6 110.6 g
Reaction Conditions:
with Aluminum Chloride in 1,2-dichloro-benzene at 50 - 55; for 2.5 h;Inert atmosphere;Solvent;Temperature;
Steps:
5.1; 5.2 Example 5.2:
A solution of 1-(2,4-dichlorophenyl)cyclopropanecarbaldehyde (109.9 g, 0.5 mol) in 1,2-dichlorobenzene(64.8 g, 0.44 mol) was added over 90 minutes to a suspension of AICI3 (87.6 g, 0.65 mol) in 1,2-dichlorobenzene (100.0 g, 0.68 mol) at Ti = 50-55 °C; the suspension was purged with a light stream of nitrogen during the dosing and the post addition stirring period. Essentially complete conversion was achieved after a post addition stirring period of 1 h at Ti = 50-55 °C. The reaction mixture was cooled to rt and then dosed onto a 14% aqueous HCI-solution (352.7 g, 1.34 mol) in a way that Ti was kept below 40 °C. The mixture was stirred for 45 minutes before the phases were separated. The organic phasewas subsequently extracted twice with water (83.3 g, 4.6 mol and 80.0 g, 4.4 mol) before the organic phase was concentrated under reduced pressure. 2-(2,4-dichlorophenyl)cyclobutanone (110.6 g) was isolated as a brown oil.1H NMR(400MHz, CDCI3)o 7.40 (d, J=2.2Hz, 1H), 7.30(d, J=8.lHz, 1H), 7.21 (dd, J=2.2 and 8.4Hz, 1H), 4.76 (m, 1H), 3.25 (m, 1H), 3.10 (m, 1H), 2.63 (m, 1H), 2.12 (m, 1H).
References:
WO2019/96860,2019,A1 Location in patent:Page/Page column 26; 27
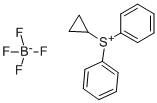
33462-81-6
141 suppliers
$85.00/1g
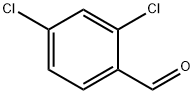
874-42-0
436 suppliers
$6.00/25g

1261038-31-6
9 suppliers
inquiry
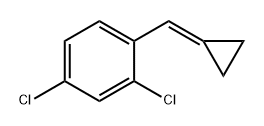
1261038-27-0
0 suppliers
inquiry

1261038-31-6
9 suppliers
inquiry

874-42-0
436 suppliers
$6.00/25g

1261038-31-6
9 suppliers
inquiry
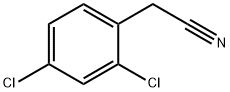
6306-60-1
253 suppliers
$14.00/5g

1261038-31-6
9 suppliers
inquiry