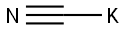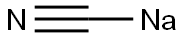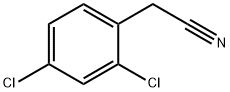
2,4-Dichlorophenylacetonitrile synthesis
- Product Name:2,4-Dichlorophenylacetonitrile
- CAS Number:6306-60-1
- Molecular formula:C8H5Cl2N
- Molecular Weight:186.04
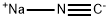
773837-37-9
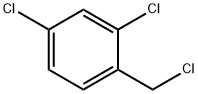
94-99-5

6306-60-1
General method: 2,4-dichlorobenzyl chloride (1.0 mmol) was mixed with sodium cyanide (2 mmol) in deionized water (5 mL) and polyethylene glycol modified diisocyanate (PEG-MDIL, 0.2 g) was added. The reaction suspension was placed under reflux conditions and the reaction mixture was stirred using a magnetic stirrer for the reaction time referred to in Table 2.After completion of the reaction, the mixture was extracted with ether (2 × 10 mL). The organic extracts were combined, dried with anhydrous calcium chloride and subsequently concentrated under reduced pressure. By this method, the target product 2,4-dichlorophenylacetonitrile was obtained in good to excellent isolated yields (see Scheme 3).
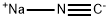
773837-37-9
0 suppliers
inquiry
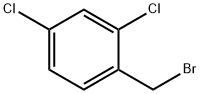
20443-99-6
111 suppliers
$25.00/1g

6306-60-1
253 suppliers
$14.00/5g
Yield:6306-60-1 93%
Reaction Conditions:
in ethanol;water
Steps:
8 Example 8
To a 100 mL round bottom flask was added 2,4-dichlorobenzyl bromide(24 g, 0.1 mol) and ethanol (30 mL) and water (5 mL)Subsequently, sodium cyanide (4.9 g, 0.1 mol) was added and the reaction was stirred. After completion of the reaction, the resulting organic phase was separated and distilled to give 17.30 g of 2,4-dichlorophenylacetonitrile in 93% yield.
References:
Zhejiang Yongtai Pharmaceutical Co., Ltd.;Jin Yizhong;He Renbao;Zhou Guobin;Xie Jianren CN106117028, 2016, A Location in patent:Paragraph 0059; 0060
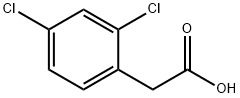
19719-28-9
399 suppliers
$7.00/10g

6306-60-1
253 suppliers
$14.00/5g