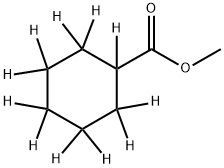
Cyclohexanecarboxylic Acid Methyl Ester-d11 synthesis
- Product Name:Cyclohexanecarboxylic Acid Methyl Ester-d11
- CAS Number:1215077-49-8
- Molecular formula:C8H14O2
- Molecular Weight:142.2
93131-16-9
24 suppliers
$140.00/50mg

74-88-4
356 suppliers
$15.00/10g

1215077-49-8
8 suppliers
inquiry
Yield:1215077-49-8 99%
Reaction Conditions:
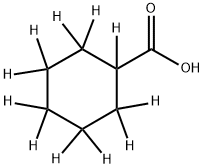
Stage #1: [1,2,2,3,3,4,4,5,5,6,6-2H11]cyclohexanecarboxylic acidwith potassium hydroxide in dimethyl sulfoxide at 20; for 0.416667 h;
Stage #2: methyl iodide in dimethyl sulfoxide at 20; for 20 h;
Steps:
51.1
Step 1: To a solution of perdeuterocyclohexanecarboxylic acid (1.71 g, 12.3 mmol) in anhydrous DMSO was added finely powdered KOH (0.729 g, 13.0 mmol). The mixture was stirred at room temperature for 25 min and methyl iodide (1.96 g, 13.8 mmol) was added. The reaction mixture was stirred at room temperature for 20 hrs and partitioned between water and Et2O. Organic layer was washed with brine, treated with activated charcoal, dried over anhydrous MgSO4 and concentrated under reduced pressure to give methyl perdeuterocyclohexanecarboxylate as a colorless oil. Yield (1.86 g, 99%); 1H NMR (400 MHz, DMSO-d6) δ 3.55 (s).
References:
US2010/93865,2010,A1 Location in patent:Page/Page column 113

67-56-1
790 suppliers
$9.00/25ml

93131-16-9
24 suppliers
$140.00/50mg

1215077-49-8
8 suppliers
inquiry