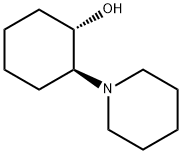
Cyclohexanol, 2-(1-piperidinyl)-, (1S,2S)- synthesis
1synthesis methods
- Product Name:Cyclohexanol, 2-(1-piperidinyl)-, (1S,2S)-
- CAS Number:174293-30-2
- Molecular formula:C11H21NO
- Molecular Weight:183.29
Yield:174293-30-2 96%
Reaction Conditions:
with ferrocenium(III) tetrafluoroborate in neat (no solvent) at 60; for 6 h;regioselective reaction;
Steps:
β-Amino Alcohols; General Procedure
General procedure: Catalyst Fe(Cp)2BF4 (5 mol%) was added to the cooled epoxide (2 mmol) at 0-5 °C and then amine (2.1 mmol) was added. The resulting mixture was stirred at 25 °C for the specified time (TLC monitoring). When the reaction was complete, the crude product was purified by column chromatography. For entry 9 in Table 1, CH2Cl2(0.5 mL) was used as solvent.
References:
Yadav, Geeta Devi;Chauhan, Manmohan Singh;Singh, Surendra [Synthesis,2014,vol. 46,# 5,p. 629 - 634]