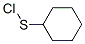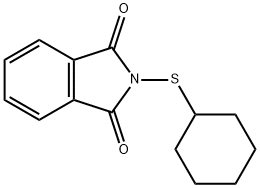
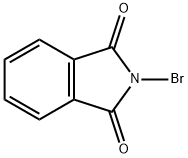
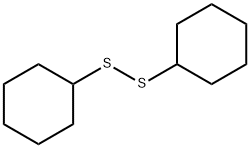
Cyclohexylthiophthalimide synthesis
- Product Name:Cyclohexylthiophthalimide
- CAS Number:17796-82-6
- Molecular formula:C14H15NO2S
- Molecular Weight:261.34
2439-85-2
2550-40-5

17796-82-6
Example 1: Synthesis of 2-(cyclohexylthio)isoindoline-1,3-dione 1,2-Dicyclohexyl disulfide (0.51 g, 2.21 mmol) was dissolved in anhydrous acetonitrile (20 mL) with N-bromophthalimide (0.50 g, 2.21 mmol) in a microwave reactor. The microwave irradiation reaction was carried out at 100 °C and 1 bar pressure for 10 min. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The resulting residue was purified by fast column chromatography with the eluent of hexane/ethyl acetate (2:1, v/v) to afford the target compound 2-(cyclohexylsulfanyl)isoindoline-1,3-dione as a light yellow solid (0.29 g, 52% yield). 1H NMR (CDCl3) δ: 7.95-7.91 (2H, dd, 3JHH = 5.46 Hz, 4JHH = 3.07 Hz), 7.81-7.76 (2H, dd, 3JHH = 5.46 Hz, 4JHH = 3.07 Hz), 3.22-3.15 (1H, tt, 3JHH = 3.63 Hz, 10.88 Hz), 1.95-1.88 (2H, m), 1.84-1.75 (2H, m), 1.65-1.57 (1H, m), 1.43-1.33 (2H, m), 1.32-1.18 (3H, m).

2439-85-2
151 suppliers
$15.00/5g

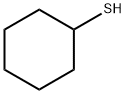
2550-40-5
131 suppliers
$14.00/25mL

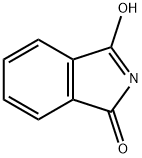
17796-82-6
253 suppliers
$5.00/10g
Yield:17796-82-6 52%
Reaction Conditions:
in acetonitrile at 100; under 750.075 Torr; for 0.166667 h;Microwave irradiation;
Steps:
1
Example 1: Synthesis of 2-(Cyclohexylthio)isoindoline-l,3-dione.; The following compound was used as an intermediate product in the synthesis of sulfenyl guanidine prodrugs of metformin.Dicyclohexyl disulphide (0.51 g, 2.21 mmol) and iV-bromophthalimide (0.50 g, 2.21 mmol) in anhydrous ACN (20 ml) were irradiated at 100 0C (1 bar) in a microwave reactor for 10 min. The solvent was evaporated under reduced pressure and the residue was purified by flash chromatography eluting with hexane/ethyl acetate (2:1) solution to obtain the compound 1 as a light yellow solid (0.29 g, 52%).1H NMR (CDCl3): δ 7.95-7.91 (2H, dd, 3JHH = 5.46 Hz, 4JHH = 3.07 Hz), 7.81-7.76 (2H, dd, 3JHH = 5.46 Hz, 4JHH = 3.07 Hz), 3.22-3.15 (IH, tt, 3JHH = 3.63, 10.88 Hz), 1.95-1.88 (2H, m), 1.84-1.75 (2H, m), 1.65-1.57 (IH, m), 1.43-1.33 (2H, m), 1.32-1.18 (3H, m).
References:
UNIVERSITY OF EASTERN FINLAND;HUTTUNEN, Kristiina;LEPPÄNEN, Jukka;RAUTIO, Jarkko;VEPSÄLÄINEN, Jouko WO2010/100337, 2010, A1 Location in patent:Page/Page column 6-7
136918-14-4
0 suppliers
inquiry
1569-69-3
126 suppliers
$33.29/25g

17796-82-6
253 suppliers
$5.00/10g

136918-14-4
0 suppliers
inquiry

2550-40-5
131 suppliers
$14.00/25mL

17796-82-6
253 suppliers
$5.00/10g
103-83-3
350 suppliers
$14.00/5g

136918-14-4
0 suppliers
inquiry

2550-40-5
131 suppliers
$14.00/25mL

17796-82-6
253 suppliers
$5.00/10g