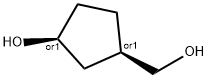
Cyclopentanemethanol, 3-hydroxy-, (1R,3S)-rel- synthesis
- Product Name:Cyclopentanemethanol, 3-hydroxy-, (1R,3S)-rel-
- CAS Number:111292-52-5
- Molecular formula:C6H12O2
- Molecular Weight:116.16
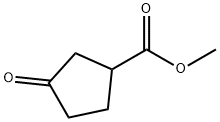
32811-75-9
163 suppliers
$90.00/500mg

111292-52-5
6 suppliers
inquiry
Yield:111292-52-5 75%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 1 h;Inert atmosphere;
Steps:
Step B: 3-(hydroxymethyl)cyclopentanol
To a solution of LiAlH4 (721 mg, 18.99 mmol, 3 eq) in THF (20 mL) was added dropwise a solution of methyl 3-oxocyclopentanecarboxylate (900 mg, 6.33 mmol, 1 eq) in THF (5 mL) at 0 °C under N2. The mixture was stirred at 0 °C for 1 h. The mixture was warmed to 20 °C and stirred for 12 h. The reaction mixture was diluted with THF (20 mL), and then the mixture was quenched with sodium sulfate decahydrate (1 g). The mixture was filtered and the filtrate was concentrated in vacuum. The residue was purified by FC (PE : EtOAc 3:1 to 0:1) to give the title compound (550 mg, 75% yield) as a yellow oil. 1H NMR (DMSO-d6): d 4.43-4.38 (m, 2 H), 4.05-4.02 (m, 1 H), 3.33-3.31 (m, 1 H), 3.25- 3.22 (m, 1 H), 1.96-1.81 (m, 2 H), 1.61-1.55 (m, 2 H), 1.47-1.36 (m, 2 H) and 1.16-1.09 (m, 1 H).
References:
WO2021/32591,2021,A1 Location in patent:Page/Page column 147-148
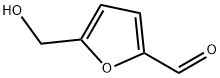
67-47-0
571 suppliers
$5.00/100mg

111292-52-5
6 suppliers
inquiry
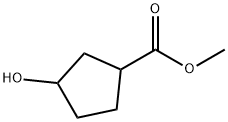
32811-76-0
31 suppliers
inquiry

111292-52-5
6 suppliers
inquiry
98-78-2
233 suppliers
$10.00/250mg

111292-52-5
6 suppliers
inquiry

1100829-91-1
0 suppliers
inquiry

111292-52-5
6 suppliers
inquiry