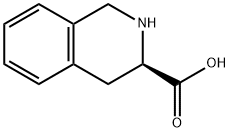
D-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid synthesis
- Product Name:D-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid
- CAS Number:103733-65-9
- Molecular formula:C10H11NO2
- Molecular Weight:177.2

50-00-0
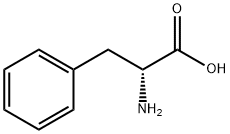
673-06-3

103733-65-9
General procedure for the synthesis of (R)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid from formaldehyde and D-phenylalanine: 1. Preparation of (3R)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid: D-phenylalanine (50 g, 0.3 mol), concentrated hydrochloric acid (386 mL), and 37% wt formalin (113.7 mL) were mixed, and the reaction was carried out for 4 h at 95 °C with vigorous stirring. Upon completion of the reaction, the mixture was cooled to room temperature and stirring was continued for 2 hours. The reaction mixture was filtered and the precipitate was washed with cold water to afford (3R)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (25 g, 42% yield) in white solid form. 2. Esterification reaction: benzyl alcohol (64 g) and p-toluenesulfonic acid (26.9 g) were added to the above product, and the reaction was carried out under reflux in a Dean-Stark apparatus with benzene (400 mL) as solvent. After completion of the reaction, the solvent was removed in vacuum and the crude product was ground with ether to obtain a solid, which was subsequently recrystallized with water and methanol to give (R)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (28 g, 35% yield) in white solid form.
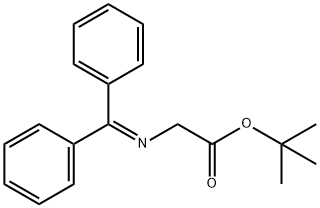
81477-94-3
266 suppliers
$6.00/1g
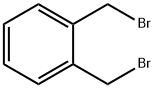
91-13-4
252 suppliers
$14.00/1g

103733-65-9
253 suppliers
$5.00/100mg
Yield:103733-65-9 82%
Reaction Conditions:
Stage #1: N-(diphenylmethylene)glycine tert-butyl ester;α,α'-dibromo-o-xylenewith (S,S)-3,4,5-F3-Ph-NAS-Br in potassium hydroxide;toluene at 0; for 6 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;
Stage #3: with sodium hydrogencarbonate
References:
Ooi;Takeuchi;Maruoka [Synthesis,2001,# 11,p. 1716 - 1718]

103733-65-9
253 suppliers
$5.00/100mg
115-11-7
243 suppliers
$45.00/25g

103733-65-9
253 suppliers
$5.00/100mg