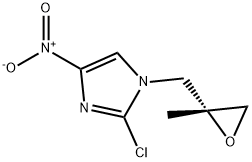
DE-7 synthesis
- Product Name:DE-7
- CAS Number:681490-93-7
- Molecular formula:C7H8ClN3O3
- Molecular Weight:217.61
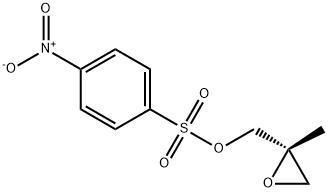
683276-63-3
13 suppliers
inquiry
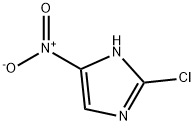
57531-37-0
221 suppliers
$7.00/100mg

681490-93-7
25 suppliers
inquiry
Yield: 64.1%
Reaction Conditions:
with potassium carbonate in DMF (N,N-dimethyl-formamide) at 20 - 50; for 9 h;
Steps:
7 Preparation of (S)-2-chloro-1-(2-methyl-2-oxiranylmethyl)-4-nitroimidazole
Example 7; Preparation of (R)-2-chloro-1-(2-methyl-2-oxiranylmethyl)-4-nitroimidzole; To N,N-dimethylformamide (100 ml) solution of (S)-2-methylglycidyl-4-nitrobenzenesulfonate (50.0 g, 97.8% e.e.) was added 2-chloro-4-nitroimidazole (26.99 g) and potassium carbonate (27.82 g) at a room temperature. After being stirred this reaction mixture at 50°C for 9 hours, the mixture was cooled to a room temperature, then ethyl acetate (150 ml) was added, after being removed the insoluble matters by filtration, the mixture was washed with water and an aqueous solution being saturated with sodium chloride in this order. Ethyl acetate layer was dried over anhydrous magnesium sulfate, then was concentrated under a reduced pressure to obtain pale brown solid product (38.2 g). The thus obtained pale brown solid was dissolved in toluene (380 ml), next silica gel (7.6 g) was added, after being stirred at a room temperature, the silica gel was removed by filtration. This treatment was repeated twice, then the mother liquor was concentrated, the residue was crystallized by adding diisopropyl ether, there was obtained (R)-2-chloro-1-(2-methyloxiranylmethyl)-4-nitroimidazole (25.54 g, yield: 64.1%) as pale yellow crystals. Melting point: 65.5 - 67.0°C1H-NMR (CDCl3) δ(ppm): 1.39 (3H, s), 2.62 (1H, d, J=3.6Hz), 2.79 (1H, d, J=3.6Hz), 3.99 (1H, d, J=14.7Hz), 4.38 (1H, d, J=14.7Hz), 7.87 (1H, s). Optical purity: 95.9% e.e. The optical purity was determined by use of a high performance liquid chromatography (HPLC) under the following conditions. Column: CHIRALPAK AD (4.6 mmφ× 250 mm) [manufactured by Daicel Chemical Industries, Ltd.]Moving bed: n-hexane/ethanol = 850/150Flow velocity: 1.0 ml/minuteDetection wave length: 254 nm.
References:
Otsuka Pharmaceutical Company, Limited EP1553088, 2005, A1 Location in patent:Page/Page column 50-51
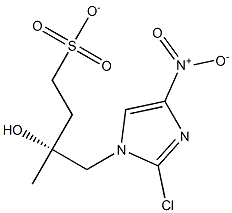
681490-92-6
7 suppliers
inquiry

681490-93-7
25 suppliers
inquiry

681490-91-5
8 suppliers
inquiry

681490-93-7
25 suppliers
inquiry
![(R)-2-chloro-1-[2-hydroxy-2-methyl-3-(4-nitrobenzoyloxy)]-propyl-4-nitroimidazole](/CAS/20180703/GIF/681490-95-9.gif)
681490-95-9
8 suppliers
inquiry

681490-93-7
25 suppliers
inquiry