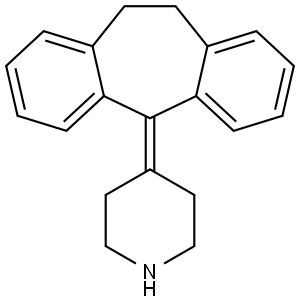
Desloratadine Impurity synthesis
- Product Name:Desloratadine Impurity
- CAS Number:50603-12-8
- Molecular formula:C20H21N
- Molecular Weight:275.39
Yield:50603-12-8 71%
Reaction Conditions:
with titanium tetrachloride;zinc in tetrahydrofuran at 0 - 75; for 2 h;
Steps:
4-(10,11-dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)piperidine
A solution of dibenzosuberone (0.82 mL, 4.56 mmol) and N-Boc-4-piperidone (1.00 g, 5.02 mmol) in THF (10.0 mL), was treated with Zn powder (1.34 g, 20.52 mmol), cooled to 0 C, and treated slowly and dropwise with TiCl4 (1.13 mL, 10.26 mmol) (WO 1998038166 A1, 1998, ref. 42). The mixture was warmed to 25 C, stirred for 30 min, heated to 75 C for 90 minutes, and then cooled to 25 C. The mixture was diluted in toluene (50 mL) and then treated with 1 M aqueous HCl (5.0 mL). The organic layer was separated and the aqueous layer was extracted with toluene (2 50 mL). The combined organic layers were concentrated in vacuo. The residue was dissolved in a minimal amount of toluene and purified by flash chromatography (SiO2, 0-20% ethyl acetate-hexanes, followed by 17:2:1 CH2Cl2:MeOH:NH4OH) to afford the title compound (0.886 g, 71%) as a white solid. 1H NMR (600 MHz, CDCl3) 7.12-7.18 (4H, m), 7.10-7.13 (2H, m), 7.07-7.11 (2H, m), 3.42-3.46 (2H, m), 2.97-3.00 (2H, m), 2.82-2.87 (2H, m), 2.72-2.77 (2H, m), 2.36-2.38 (4H, m); 13C NMR (150 MHz, CDCl3) 140.9, 138.1, 134.9, 134.6, 129.4, 129.0, 126.9, 125.6, 48.5, 32.7, 32.6; LCMS m/z 276.3 ([M + H+], C25H29NO2 requires 276.2).
References:
Kastrinsky, David B.;Sangodkar, Jaya;Zaware, Nilesh;Izadmehr, Sudeh;Dhawan, Neil S.;Narla, Goutham;Ohlmeyer, Michael [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 19,art. no. 12436,p. 6528 - 6534] Location in patent:supporting information
1210-35-1
299 suppliers
$6.00/1g

50603-12-8
14 suppliers
inquiry
63463-36-5
16 suppliers
inquiry

50603-12-8
14 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-, ethyl ester](/CAS/20210111/GIF/134204-86-7.gif)
134204-86-7
0 suppliers
inquiry

50603-12-8
14 suppliers
inquiry
5570-77-4
342 suppliers
$14.00/5g

50603-12-8
14 suppliers
inquiry
