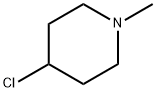
4-Chloro-N-methylpiperidine synthesis
- Product Name:4-Chloro-N-methylpiperidine
- CAS Number:5570-77-4
- Molecular formula:C6H12ClN
- Molecular Weight:133.62
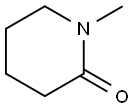
931-20-4

103057-10-9

5570-77-4
To an anhydrous tetrahydrofuran (THF, 7 mL) solution of diisopropylamine (1.078 mL) was slowly added n-butyllithium (3.08 mL, 2.5 M) dropwise at 0 °C. The reaction solution was stirred at 0 °C for 40 min and then cooled to -23 °C. Subsequently, N-methyl-2-piperidone (0.80 mL) was added to the reaction system and stirred at -23°C for 0.5 hr, after which stirring was continued at -78°C for 1 hr. Next, a solution of anhydrous THF (14 mL) of 4-chloromethyl-N-tritylimidazole (2.70 g) was added dropwise to the above mixture. The reaction mixture was stirred at -78 °C for 4 hours, followed by slow warming to room temperature and stirring overnight (16 hours). Upon completion of the reaction, water and ethyl acetate were added to the mixture and the organic layer was separated after vigorous shaking. The aqueous layer was extracted several times with ethyl acetate and the combined organic layers were washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated to give the crude product. The crude product was purified by fast chromatography (eluent: dichloromethane solution of saturated methanol with 1% to 2% ammonia) to afford the target compound N-methyl-4-chloropiperidine (1.58 g, 52% yield).
106-52-5
344 suppliers
$6.00/25g

5570-77-4
342 suppliers
$14.00/5g
Yield:5570-77-4 80%
Reaction Conditions:
with thionyl chloride;triethylamine in dichloromethane at 20 - 40; for 2 h;
Steps:
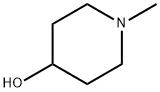
4.1 Synthesis of N-methylpiperidine-4-boronic acid
The first step: in the reaction flask, N-methylpiperidin-4-ol (115.2g, 1mol),Dissolve triethylamine (506.0g, 5mol) in dichloromethane (955.0g), stir well, control the temperature from 20°C to 40°C ,Thionyl chloride (234.9g, 2mol) was added dropwise, after the dropwise addition, the reaction was stirred at 40°C for 2 hours,After the reaction is detected by GC, add water (1000.0g) to quench the reaction,The water layer was extracted twice with dichloromethane (250.0g*2 times), the organic layers were combined, and the organic solvent was evaporated to dryness first.Then use an oil pump to distill 106.9g of light yellow liquid product N-methylpiperidine-4-chloride under reduced pressure.HPLC purity>98%, yield 80%.
References:
Dalian Shuangpeng Pharmaceutical And Chemical Co., Ltd.;Sun Fuyuan;Zheng Peng CN111171063, 2020, A Location in patent:Paragraph 0027

931-20-4
62 suppliers
$183.00/10g

103057-10-9
45 suppliers
$45.00/50mg

5570-77-4
342 suppliers
$14.00/5g
5382-23-0
175 suppliers
$19.69/25g

5570-77-4
342 suppliers
$14.00/5g
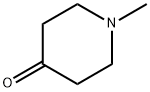
1445-73-4
441 suppliers
$15.00/25g

5570-77-4
342 suppliers
$14.00/5g
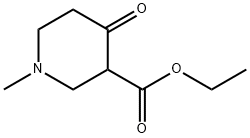
25012-72-0
33 suppliers
$65.00/50mg

5570-77-4
342 suppliers
$14.00/5g