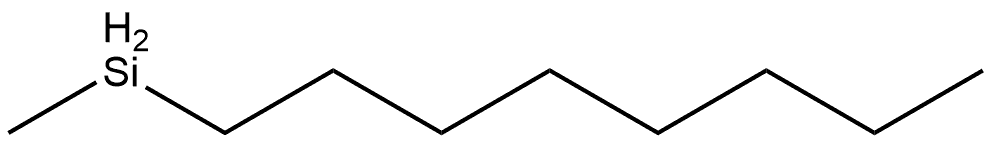
Di-n-Octylmethylsilane synthesis
- Product Name:Di-n-Octylmethylsilane
- CAS Number:51502-63-7
- Molecular formula:C17H38Si
- Molecular Weight:270.57
Yield:51502-63-7 96.4%
Reaction Conditions:
Stage #1: 1-bromo-octanewith magnesium in tetrahydrofuran at 20; for 3.5 h;Inert atmosphere;
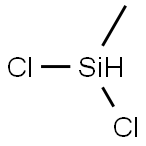
Stage #2: Dichloromethylsilane in tetrahydrofuran at 0 - 20;
Steps:
Typical procedure for the synthesis of 5(m,m) (m = n) Methyldioctylsilane 5(8,8)
Under nitrogen, 1-bromooctane (10.6 g, 54.9 mmol) was added dropwise to Mg (1.46 mg,60.1 mmol) in dry THF (60 mL) at rt over 30 min and stirred for 3 h. To the solution was added dichloromethylsilane (2.50 mL, 24.3 mmol) dropwise at 0 °C over 10 min, warmed up to room temperature and stirred for 2 h. After adding 1N HCl aq. to quench the reaction, the precipitate was removed by filtration and the filtrate was concentrated to remove THF. The residue was dissolved in hexane and washed with water. The organic layer was dried over anhyd. Na2SO4 and concentrated under reduced pressure to give a pale yellow liquid. The crude product was distilled by Kugelrohr (< 10 mmHg, 180 °C) to afford a colorless liquid (6.34 g, 23.4 mmol, 96.4%). 1H NMR (300 MHz, CDCl3): δ (ppm) = 3.75(oct, J = 3.6 Hz, 1H, Si-H), 1.38-1.23 (m, 24H, -CH2-), 0.88 (t, J = 6.9 Hz, 6H, -CH3), 0.62-0.52 (m, 4H, Si-CH2-), 0.03 (d, J =3.6 Hz, 3H, Si-CH3); IR(neat): νmax (cm-1)= 2958, 2924, 2854, 2106, 1466, 1251, 880, 721.
References:
Hirose, Takuji;Miyazaki, Yutaro;Watabe, Mizuki;Akimoto, Sho;Tachikawa, Tatsuya;Kodama, Koichi;Yasutake, Mikio [Tetrahedron,2015,vol. 71,# 29,p. 4714 - 4721] Location in patent:supporting information

111-66-0
173 suppliers
$10.00/10ml
80204-10-0
0 suppliers
inquiry

51502-63-7
12 suppliers
inquiry

40934-68-7
16 suppliers
$495.44/5MG