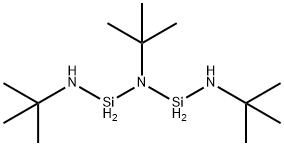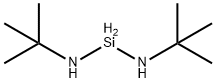
DI(T-BUTYLAMINO)SILANE synthesis
- Product Name:DI(T-BUTYLAMINO)SILANE
- CAS Number:186598-40-3
- Molecular formula:C8H22N2Si
- Molecular Weight:174.36
Yield: 31.2 g , 107.5 g
Reaction Conditions:
with Dichlorosilane in tert-butyl methyl ether at -40 - 40; for 29.5 h;
Steps:
5 Example 5: Synthesis of 1.3.5-Tri-(tei1-butyr)-trisilazane
Example 5: Synthesis of 1.3.5-Tri-(tei1-butyr)-trisilazane CI t-Bu 3 H- Si C I + 9 tBuNH2 (t-BuNH)2SiH2 + t-Bu H-SiH2-N-SiH2-NHt-Bu H [0036] Under an argon atmosphere, a 5-liter 4-necked flask equipped with a cooling bath, overhead stirrer, pot thermometer, sub-surface dip-tube, and dry-ice condenser was charged with methyl t-butyl ether (1 144.3g). The mixture was cooled to -40°C and dichlorosilane (2.5 mol, 252.5g) was slowly added to the pot. t-Butylamine (7.5 mol 548.6g) was then added via dip-tube between -30 to -20°C over 2.5 hours. After addition was completed, the reaction mixture was slowly warmed to 25°C and stirred at this temperature for 24 hours. Additional t-Butylamine (3.75 mol, 274.3g) was added to the reaction mixture between 0 and 40°C. The mixture was stirred for 3 hours and monitored by GC. The reaction mixture was filtered and solvents were removed from the filtrates under reduced pressure below 50°C. The reaction mixture was filtered again and fractional distillation of the clear filtrates afforded 107.5 g (14.0 mol) of Bis(t-butylamino)silane and 31.2 g of l,3,5-Tri-(tcrt-butyl)-trisilazane: b.p. 62-3uC/0.3mmHg, density20°C:0.868, FTIR: vS- H:2140.9(vs) and lH MR(CDC13):1.19(s, 18H), 1.37(s, 9H ) and 4.73(s, 4H).
References:
GELEST TECHNOLOGIES, INC.;ARKLES, Barry, C.;PAN, Youlin;JOVE, Fernando WO2016/153929, 2016, A1 Location in patent:Page/Page column 11
75-64-9
450 suppliers
$10.00/10g

186598-40-3
45 suppliers
$72.00/1g