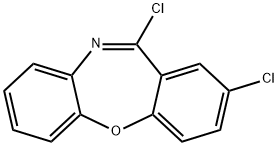
Dibenz[b,f][1,4]oxazepine, 2,11-dichloro- synthesis
- Product Name:Dibenz[b,f][1,4]oxazepine, 2,11-dichloro-
- CAS Number:3455-14-9
- Molecular formula:C13H7Cl2NO
- Molecular Weight:264.11
![2-Chlorodibenz[b,f][1,4]oxazepin-11(10H)-one](/CAS/GIF/3158-91-6.gif)
3158-91-6
141 suppliers
$39.60/250mg
![Dibenz[b,f][1,4]oxazepine, 2,11-dichloro-](/CAS/20150408/GIF/3455-14-9.gif)
3455-14-9
14 suppliers
inquiry
Yield:3455-14-9 33%
Reaction Conditions:
with N,N-dimethyl-aniline;trichlorophosphate in toluene at 95; for 2.5 h;
Steps:
A 25 mL flask, equipped with stir bar, was charged with 2- chlorodibenzo[b,f][l,4]oxazepin-l l(10H)-one (100 mg, 0.4 mmol), YV-dimethylaniline (0.21 mL, 1.6 mmol), POCI3(114 pL, 1.14 mmol), and toluene (4 mL). The resulting suspension was SL-1511 heated to 95°C for 2.5 hours, and a dark brown solution formed.Solvent was removed under reduced pressure, and the residue dissolved in in a mixture of dioxane (2 mL) and aqueous 2M Na2C03(3 mL). The resulting solution was heated at 80°C for 50 minutes, dioxane removed under reduced pressure, and the residue extracted in EtOAc (3 x 10 mL). Combined EtOAc solutions were dried over MgS04, filtered, and the solvent removed under reduced pressure. The residue was purified by silica gel chromatography (0 30% EtOAc/hexanes) to provide 35 mg (33% yield) of imidoyl chloride SL-1511 as a white solid. NMR (400 MHz, CDCb) d 7.71 (d, J = 2.6 Hz, 1H), 7.47 (dd, J = 8.7, 2.5 Hz, 1H),7.33 (dd, J = 7.5, 2.1 Hz, 1H), 7.26 (td, J = 7.5, 1.7 Hz, 1H, overlap with CHC13), 7.21 (td, J = 7.5, 1.7 Hz, 1H), 7.15 (dd, J = 7.6, 1.7 Hz, 1H), 7.13 (d, J = 8.7 Hz, 1H); MS (ESI) calc’d for CisHsChNO [M+H]+264.00 found 263.87.
References:
WO2020/117954,2020,A2 Location in patent:Page/Page column 17; 56; 57
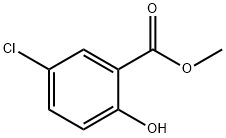
4068-78-4
199 suppliers
$12.00/5g
![Dibenz[b,f][1,4]oxazepine, 2,11-dichloro-](/CAS/20150408/GIF/3455-14-9.gif)
3455-14-9
14 suppliers
inquiry
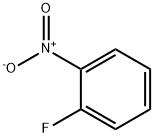
1493-27-2
510 suppliers
$5.00/10g
![Dibenz[b,f][1,4]oxazepine, 2,11-dichloro-](/CAS/20150408/GIF/3455-14-9.gif)
3455-14-9
14 suppliers
inquiry
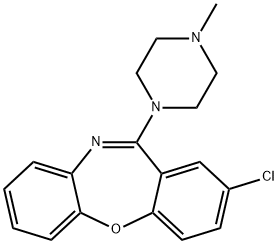
1977-10-2
45 suppliers
inquiry
![Dibenz[b,f][1,4]oxazepine, 2,11-dichloro-](/CAS/20150408/GIF/3455-14-9.gif)
3455-14-9
14 suppliers
inquiry