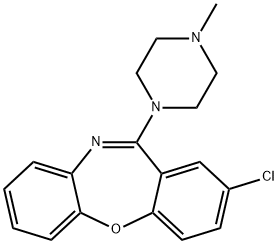
loxapine
- CAS No.
- 1977-10-2
- Chemical Name:
- loxapine
- Synonyms
- 2-Chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4]oxazepine;C07104;Loxapine, 10 mM in DMSO;Dibenz[b,f][1,4]oxazepine, 2-chloro-11-(4-methyl-1-piperazinyl)-;Inhibitor,schizophrenia,IL-2,Loxapine,inhibit,5-HT Receptor,5-hydroxytryptamine Receptor,Dopamine Receptor,Serotonin Receptor,serotonin,IL-1β,antipsychotics
- CBNumber:
- CB1875090
- Molecular Formula:
- C18H18ClN3O
- Molecular Weight:
- 327.81
- MDL Number:
- MOL File:
- 1977-10-2.mol
- MSDS File:
- SDS
| Product description | Number | Pack Size | Price |
| Loxapine | B1001 | 100mg | $84 |
| Loxapine 99.49% | CS-1105 | 100mg | $84 |
| LOXAPINE 95.00% | API0006785 | 100MG | $203.7 |
| Loxapine | B1001 | 500mg | $288 |
| Loxapine 99.49% | CS-1105 | 500mg | $288 |
| Melting point | 109-110° |
|---|---|
| Density | 1.2299 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| color | Light yellow to yellow |
| CAS DataBase Reference | 1977-10-2 |
| FDA UNII | LER583670J |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501 |
| Hazardous Substances Data | 1977-10-2(Hazardous Substances Data) |
| Toxicity | LD50 orally in mice: 65 mg/kg (Stille) |
loxapine price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ApexBio Technology | B1001 | Loxapine | 1977-10-2 | 100mg | $84 | 2021-12-16 | Buy |
| ChemScene | CS-1105 | Loxapine 99.49% | 1977-10-2 | 100mg | $84 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0006785 | LOXAPINE 95.00% | 1977-10-2 | 100MG | $203.7 | 2021-12-16 | Buy |
| ApexBio Technology | B1001 | Loxapine | 1977-10-2 | 500mg | $288 | 2021-12-16 | Buy |
| ChemScene | CS-1105 | Loxapine 99.49% | 1977-10-2 | 500mg | $288 | 2021-12-16 | Buy |
loxapine Chemical Properties,Uses,Production
Originator
Loxitane,Lederle,US,1976
Uses
Tranquilizer (minor).
Definition
ChEBI: Loxapine is a dibenzooxazepine. It has a role as an antipsychotic agent and a dopaminergic antagonist.
Manufacturing Process
One route is described in US Patent 3,412,193 as follows. To a mixture of o-
(p-chlorophenoxy)aniline hydrochloride (prepared from 32 g of the base) in 50
ml of pyridine is added gradually while heating under reflux, 25 ml of ethyl
chloroformate. After the addition is completed, the mixture is heated under
reflux for one hour longer, and then evaporated under reduced pressure to an
oily residue. The residue is taken up in 300 ml of water, and extracted with
ether (approximately 200 ml).
The ether extract is separated, dried over sodium sulfate, and evaporated to
an oily residue (40 g) which contains ethyl o-(p-chlorophenoxy)carbanilate
and is used without further purification. The crude ethyl o-(p-chlorophenoxy)
carbanilate is dissolved in 20 ml of benzene, and 20 ml of 1I-methylpiperazine
and a small amount of sodium methylate (approximately 25 to 50 mg) are
added. Benzene is then removed by slow distillation; and the mixture is
heated overnight under reflux (approximately 16 hours).
Evaporation under reduced pressure then gives a solid residue which is
dissolved in 400 ml of ether with heating. Concentration to half-volume under
reduced pressure produces a precipitate which is collected, washed with
petroleum ether and dried (36 g). A second crop of product is isolated from
the filtrate. This product is dissolved in 200 ml of chloroform and treated with
an excess of anhydrous hydrogen chloride. The resulting precipitate is
collected and dried at 50°C (in vacuo), and 4-methyl-2'-(p-chlorophenoxy)-1-piperazinecarboxanilide hydrochloride, MP 210° to 213°C, is thereby obtained.
A mixture of 4-methyl-2'-(p-chlorophenoxy)-1-piperazinecarboxanilide
hydrochloride (6 g), 50 ml of phosphorus oxychloride and 10 g of phosphorus
pentoxide is heated under reflux for about 24 hours, and then concentrated to
a gummy residue by evaporation under reduced pressure. This residue is
taken up in 150 ml of ether, 200 g of ice is added, and the mixture is made
basic with concentrated aqueous ammonium hydroxide. The ether layer is
separated, dried over potassium hydroxide pellets and evaporated to a solid
residue (approximately 4 g).
This crude product is dissolved in 100 ml of dilute hydrochloric acid, the acid
solution is extracted with ether, and the aqueous layer is made basic with
sodium hydroxide solution (3N) in the presence of ether (approximately 250
ml). The ether layer is separated, dried over potassium hydroxide and
evaporated to a white solid. Additional purification by repeating the formation
of the hydrochloric acid salt and reprecipitation of the base is carried out.
When purified in this manner, followed by drying at 80°C in vacuo over
phosphorus pentoxide, 2-chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f]
[1,4]oxazepine, MP 109° to 111°C, is obtained.
brand name
Loxitane-C Oral Suspension [as hydrochloride] (Wyeth-Ayerst); Loxitane Intramuscular [as hydrochloride] (Wyeth-Ayerst).
Therapeutic Function
Tranquilizer
Biological Activity
loxapine succinate is a d2dr and d4dr inhibitor, serotonergic receptor antagonist and also a dibenzoxazepine anti-psychotic agent [1].
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. Experimental teratogenic and reproductive effects. A tranquilizer. Many dbenz-azepine compounds have central nervous system effects. When heated to decomposition it emits very toxic fumes of Cl and NOx.
in vitro
loxapine was a typical neuroleptic that showed great structural and functional homology to the atypical antipsychotic clozapine. chronic loxapine treatment was usually associated with extrapyramidal symptoms (eps). loxapineexihibited an extremely strong binding affinity fordopamined4 andserotonin5-ht2receptors, suggesting that both serotonergic and dopaminergic mechanisms contributed to the antipsychotic drug action and eps associated with loxapine in the treatment of schizophrenia [1]. in frontal cortex of brain in human and bovine, in the presence of loxapine, [3h]ketanserin bound to 5-ht2 receptor with ki value of 6.2 nm and 6.6 nm, respectively. in comparing competition experiments involving the human membranes, loxapine exihibited the rank order of potency for the various receptors as follows: 5-ht2≥d4>>>d1>d2 [1]. loxapine administration at 0.2, 2 and 20 μmafter 1 and 3 days of exposure reduced il-1βand il-2 secretion by lps-activated mixed glia cultures. loxapine also decreased il-1βand il-2 secretion in lps-induced microglia cultures [2].
in vivo
chronic administration of loxapine(5 mg/kg) in rats for 4 weeks or 10 weeks significantly reduced more than 50% of serotonin (s2) receptor density. loxapine (5 mg/kg) didn’t change dopamine receptor density but greatly reduced serotonin receptor density by 47% in the brain of rats [3].
References
[1]. singh an1,barlas c,singh s,franks p,mishra rk. a neurochemical basis for the antipsychotic activity of loxapine: interactions withdopamined1,d2, d4 andserotonin5-ht2receptorsubtypes.j psychiatry neurosci.1996 jan;21(1):29-35.
[2]. labuzek k1,kowalski j,gabryel b,herman zs. chlorpromazine and loxapine reduce interleukin-1beta and interleukin-2 release by rat mixed glial and microglial cell cultures.eur neuropsychopharmacol.2005 jan;15(1):23-30.
[3]. lee t,tang sw. loxapine and clozapine decreaseserotonin(s2) but do not elevatedopamine(d2)receptornumbers in the rat brain.psychiatry res.1984 aug;12(4):277-85.
loxapine Preparation Products And Raw materials
Raw materials
Preparation Products
loxapine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354; +17819995354 | marketing@targetmol.com | United States | 32435 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 | sales@hzclap.com | CHINA | 6312 | 58 |
| HANGZHOU LEAP CHEM CO., LTD. | +86-571-87711850 | market18@leapchem.com | China | 43339 | 58 |
| Aladdin Scientific | tp@aladdinsci.com | United States | 57505 | 58 | |
| XIAMEN AMITY INDUSTRY AND TRADE CO., LTD. | +8618950047208 | ellena@amitychem.com | China | 43416 | 58 |
| Chongqing jooe co., ltd | +undefined86-15223382610 | info@jooe.com | China | 6208 | 58 |
| Beijing HuaMeiHuLiBiological Chemical | 010-56205725 | waley188@sohu.com | China | 12335 | 58 |
| Shanghai Aladdin Bio-Chem Technology Co.,LTD | 400-6206333 13167063860 | anhua.mao@aladdin-e.com | China | 25003 | 65 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4861 | 58 |
| ShangHai Caerulum Pharma Discovery Co., Ltd. | 18149758185 | sales-cpd@caerulumpharma.com | China | 3506 | 58 |