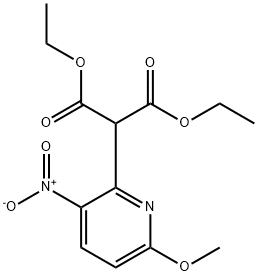
Diethyl 2-(6-Methoxy-3-nitropyridin-2-yl)Malonate synthesis
- Product Name:Diethyl 2-(6-Methoxy-3-nitropyridin-2-yl)Malonate
- CAS Number:1000342-55-1
- Molecular formula:C13H16N2O7
- Molecular Weight:312.28
Yield:1000342-55-1 80%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;mineral oil at 0 - 80; for 16 h;
Steps:
13 Typical Procedure for the Preparation of SpiroOxindoles, Exemplified by the Preparation of Intermediate 97, 5’-methoxy-4H-spiro[cyclohexane-1 ,3’-pyrrolo[3,2-b]pyridine] -2,4(1 ‘H)-dione
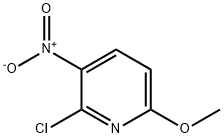
To NaR (4.24 g, 60% w/w in mineral oil, 106.0 mmol) in THF (80 mE) was added diethyl malonate (16.16mE, 106.0 mmol) dropwise at 00 C. and the reaction mixture stirred for 1 h. 2-chloro-6-methoxy-3-nitropyridine (10.00 g, 53.0 mmol) in THF (20 mE) was added dropwise and the resulting mixture stirred at 80° C. for 16 h. The reaction mixture was paititioned between cold H20 (250 mE) and EtOAc (100 mE), the aqueous layer thither extracted with EtOAc (2x100 mE), combined organics dried (Na2504) and the solvent removed in vacuo. The crude product was purified by column chromatography (normal phase silica, 0 to 20% EtOAc in hexane) to give diethyl 2-(6-methoxy-3- nitropyridin-2-yl)malonate (13.21 g, 80%) as a light green solid.
References:
US2018/105491,2018,A1 Location in patent:Paragraph 0710; 0711; 0712; 0713