Diethyl 3-fluorobenzylphosphonate synthesis
- Product Name:Diethyl 3-fluorobenzylphosphonate
- CAS Number:63909-57-9
- Molecular formula:C11H16FO3P
- Molecular Weight:246.22
Yield:63909-57-9 100%
Reaction Conditions:
at 150; for 2 h;Inert atmosphere;
Steps:
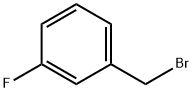
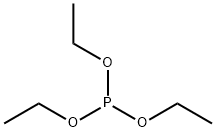
Intermediate 20Diethyl 3-fluorobenzylphosphonate. A flask was charged with 1- (bromomethyl)-3-fluorobenzene (7.5 mL, 61.1 mmol) and treated withtriethylphosphite (21.39 mL, 122 mmol) dropwise with stirring. Upon completion of the addition, the reaction was fitted with a reflux condensor (no cooling) and a slow stream of nitrogen was passed over the reaction mixture. The reaction was slowly warmed to 150°C and held there for 2 h. The reaction was cooled to room temperature and concentrated under high vacuum to remove most of the excess triethylphosphite. The resulting residue was purified by column chromatography (50% -> 100% EtO Ac/Hex) to give 15.13 g (quant.) as a colorless oil. XH-NMR (CDC13, 500 MHz) δ 7.29 (m, 1H), 7.10 (m, 1H), 7.05 (m, 1H), 6.97 (m, 1H), 4.06 (m, 4H), 3.16 (d, J=21.7, 2H), 1.28 (t, J=7.0, 6H). 13C-NMR (CDC13, 126 MHz) δ 162.85 (dd, J=246, 3.8), 134.2 (t, J=8.6), 130.0 (dd, J=7.7, 2.9), 125.6 (dd, J=6.7, 2.9), 116.9 (dd, J=22, 6.7, 114.0 (dd, J=21, 2.9), 62.3 (d, J=6.7), 33.7 (dd, J=138, 1.9, 16.5 (d, J=5.8).
References:
WO2012/64603,2012,A1 Location in patent:Page/Page column 56-57
456-42-8
327 suppliers
$5.00/5g

63909-57-9
16 suppliers
inquiry