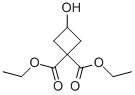
DIETHYL 3-HYDROXYCYCLOBUTANE-1,1-DICARBOXYLATE synthesis
- Product Name:DIETHYL 3-HYDROXYCYCLOBUTANE-1,1-DICARBOXYLATE
- CAS Number:99974-66-0
- Molecular formula:C10H16O5
- Molecular Weight:216.23
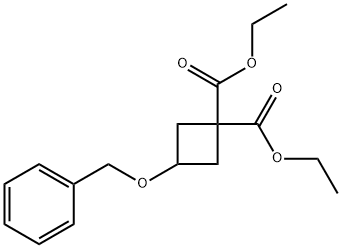
54166-15-3

99974-66-0
The general procedure for the synthesis of 3-hydroxy-1,1-cyclobutanedicarboxylic acid-1,1-diethyl ester from 3-(benzyloxy)cyclobutane-1,1-dicarboxylic acid is as follows: 3-(benzyloxy)cyclobutane-1,1-dicarboxylic acid-diethyl ester (2 g) was dissolved in ethanol (EtOH, 100 ml), and 10% palladium/carbon (Pd/C, 400 mg, 20% equiv) was added. The reaction suspension was subjected to hydrogenation at room temperature overnight. Upon completion of the reaction, the solvent was removed by evaporation under reduced pressure to afford the product 3-hydroxy-1,1-cyclobutanedicarboxylic acid-1,1-diethyl ester (1.3 g, 91.5% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3) with the following chemical shifts: δ 2.44-2.49 (m, 2H), 2.85-2.90 (m, 2H), 4.35-4.37 (m, 1H), 4.21 (q, J=7.2 Hz, 4H), 1.26 (t, J=10.8 Hz, 6H).

54166-15-3
78 suppliers
$60.00/100mg

99974-66-0
47 suppliers
$52.00/100mg
Yield:99974-66-0 97%
Reaction Conditions:
with hydrogen;palladium(II) hydroxide in ethanol;water at 20; for 8 h;
Steps:
Diethyl 3-hydroxycyclobutane-1,1-dicarboxylate (3a)
Pd(OH)2 (50% H2O, 0.516 g, 15% w/w) was added to a solution of benzyl alcohol (1.70 g, 5.55 mmol) in EtOH (20 ml) and the reaction mixture was stirred at r.t. under H2-atmosphere (60 psi) for 8 hours. The reaction mixture was filtered over Celite and the solvent was evaporated. The product was obtained as a colourless oil (1.17 g, 5.39 mmol, 97%) and used without further purification. 1H-NMR data was consistent with literature.1 1H-NMR (300 MHz, CDCl3): 1.26 (6H, t, J 7.0, OCH2CH3), 2.17 (1H, br s, OH), 2.39-2.52 (2H, m, 2/4-H), 2.82-2.94 (2H, m, 2/4-H), 4.20 (4H, q, J 7.0, OCH2CH3), 4.30-4.45 (1H, m, 3-H); 13C-NMR (125 MHz, CDCl3): 14.1 (OCH2CH3), 40.3 (C-2/4), 46.0 (C-1), 61.8 (C-3), 62.3 (OCH2CH3), 171.6 (C=O), 171.8 (C=O); LC-MS (m/z, ES+) 217.2 (30%, [M+H]+).
References:
Re?nik, Lisa-Maria;Cantelli, Christophe;Fersing, Cyril;Gongora, Céline;Pouget, Jean-Pierre;Lisowski, Vincent [Bioorganic and Medicinal Chemistry Letters,2020,vol. 30,# 22,art. no. 127527] Location in patent:supporting information

105-53-3
793 suppliers
$5.00/25g

99974-66-0
47 suppliers
$52.00/100mg