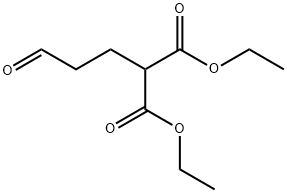
diethyl (3-oxopropyl)malonate synthesis
- Product Name:diethyl (3-oxopropyl)malonate
- CAS Number:19515-61-8
- Molecular formula:C10H16O5
- Molecular Weight:216.23
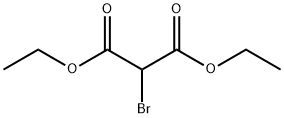
685-87-0
264 suppliers
$10.00/5g
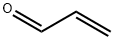
107-02-8
0 suppliers
$38.60/4s8501

19515-61-8
13 suppliers
$115.00/500 mg
Yield:-
Reaction Conditions:
with tributyl-amine in ethanol
Steps:
3.a (a)
(a) Preparation of γ,γ-dicarbethoxybutyraldehyde First, 100 ml acrolein was added to a solution of 180g diethyl bromomalonate, 14g tributylamine, and 600 ml ethanol while cooling in an ice bath. After 2-3 hours, an additional 1.5g tributylamine and 20 ml acrolein was added. The stirring was continued for an additional 1 to 2 hours without additional cooling. The reaction mixture was neutralized with 7 ml glacial acetic acid and the ethanol and unreacted acrolein were removed on a rotary evaporator. The residue was diluted with 500 ml benzene and washed with 3 * 100 ml H2 O and 2 * 100 ml saturated NaCL solution. The benzene solution was dried over CaSO4 and rotary evaporated to yield 207g of yellow oil which was indicated to be 48% product by glc.
References:
Monsanto Company US4120874, 1978, A
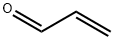
107-02-8
0 suppliers
$38.60/4s8501

105-53-3
785 suppliers
$5.00/25g

19515-61-8
13 suppliers
$115.00/500 mg
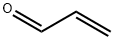
107-02-8
0 suppliers
$38.60/4s8501

105-53-3
785 suppliers
$5.00/25g

19515-61-8
13 suppliers
$115.00/500 mg

13654-26-7
0 suppliers
inquiry
35573-93-4
156 suppliers
$8.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

19515-61-8
13 suppliers
$115.00/500 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
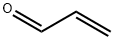
107-02-8
0 suppliers
$38.60/4s8501

19515-61-8
13 suppliers
$115.00/500 mg