
DIETHYL-6-(4-CHLOROPHENOXY) HEXYLMALONATE synthesis
- Product Name:DIETHYL-6-(4-CHLOROPHENOXY) HEXYLMALONATE
- CAS Number:82258-39-7
- Molecular formula:C19H27ClO5
- Molecular Weight:370.87
![Benzene, 1-[(6-bromohexyl)oxy]-4-chloro-](/CAS/GIF/37136-99-5.gif)
37136-99-5
6 suppliers
inquiry
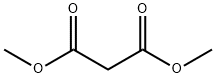
108-59-8
648 suppliers
$5.00/25g

82258-39-7
12 suppliers
$110.50/50mg
Yield:82258-39-7 61%
Reaction Conditions:
Stage #1: malonic acid dimethyl esterwith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;
Stage #2: 1-((6-bromohexyl)oxy)-4-chlorobenzene in tetrahydrofuran;mineral oil at 20; for 4 h;
Steps:
2.1.4 Diethyl 2-(6-(4-chlorophenoxy)hexyl)malonate (6) (CAS: 82258-39-7)
The diethyl 2-(6-(4-chlorophenoxy)hexyl)malonate 6 was prepared following a procedure previously reported by G. Kang et al. (2018). Sodium malonate was obtained by adding NaH (760mg, 60% NaH in mineral oil, 18.86mmol) in portions to a solution of dimethyl malonate 5 (3.18mL, 18.86mmol) in THF (40mL) at 0°C. The resulting suspension was stirred for 0.5h. The 1-((6-bromohexyl)oxy)-4-chlorobenzene 4 (5.0g, 17.15mmol) was then added in the mixture at room temperature. After completion of the reaction monitored by TLC (around 4h), the solvent was removed under reduced pressure. The residue was dissolved with saturated aqueous NH4Cl and extracted two times with DCM. The organic extracts were combined and washed with water and brine, dried over anhydrous MgSO4, filtered and concentrated in vacuo to give a brownish yellow oil. The crude was purified by silica gel column chromatography (PE/EtOAc: 20/1-10/1) to give the compound diethyl 2-(6-(4-chlorophenoxy)hexyl)malonate 6 as a light yellow oil (3.89g, 61%). Chemical Formula: C19H27ClO5, Molecular weight: 370.87g/mol. HRMS ESI: Calculated for C19H28ClO5 [M+H]+: 371.1620, found: 371.1620. 1H NMR (300MHz, CDCl3, δ ppm): 7.23 (d, J=9.0Hz, 2H), 6.82 (d, J=9.0Hz, 2H), 4.22 (q, J=9.0Hz, 4H), 3.92 (t, J=6.0Hz, 2H), 3.33 (t, J=6.0Hz, 1H), 1.96-1.88 (m, 2H), 1.80-1.75 (m, 2H), 1.40-1.32 (m, 6H), 1.28 (t, J=6.0Hz, 6H). 13C NMR (75MHz, CDCl3, δ ppm): 169.52 (2C), 157.67, 129.24 (2C), 125.30, 115.73 (2C), 68.15, 61.29, 61.00, 53.42, 52.03, 29.05, 28.96, 28.64, 27.23, 25.73, 14.10.
References:
Deskeuvre, Marine;Lan, Junjie;Dierge, Emeline;Messens, Joris;Riant, Olivier;Corbet, Cyril;Feron, Olivier;Frédérick, Rapha?l [International Journal of Pharmaceutics,2022,vol. 624,art. no. 122041]
![Benzene, 1-[(6-bromohexyl)oxy]-4-chloro-](/CAS/GIF/37136-99-5.gif)
37136-99-5
6 suppliers
inquiry

105-53-3
791 suppliers
$5.00/25g

82258-39-7
12 suppliers
$110.50/50mg

4286-55-9
308 suppliers
$15.00/10g

82258-39-7
12 suppliers
$110.50/50mg
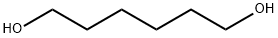
629-11-8
485 suppliers
$6.00/25g

82258-39-7
12 suppliers
$110.50/50mg