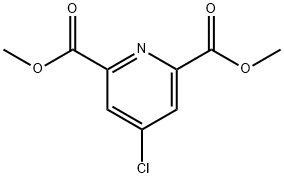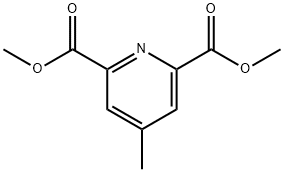
DiMethyl 4-Methylpyridine-2,6-dicarboxylate synthesis
- Product Name:DiMethyl 4-Methylpyridine-2,6-dicarboxylate
- CAS Number:4566-82-9
- Molecular formula:C10H11NO4
- Molecular Weight:209.2

67-56-1
790 suppliers
$9.00/25ml
39621-00-6
161 suppliers
$6.00/1g
201230-82-2
1 suppliers
inquiry

4566-82-9
9 suppliers
inquiry
Yield:4566-82-9 68%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;triethylamine in N,N-dimethyl-formamide at 80; under 2585.81 Torr; for 16 h;
Steps:
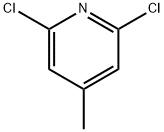
41.41-1; 57.57-1 Step 41-1 Synthesis of dimethyl 4-m ethyl pyridine-2.6-di carboxyl ate (INT 41-A)
To a solution of 2,6-dichloro-4-methyl-pyridine (1 g, 6.17 mmol) in DMF (20 mL) and MeOH (10 mL) were added Pd(dppf)Cb-CEbCb (504.05 mg, 617.22 pmol) and TEA (3.44 mL, 24.69 mmol). After stirring at 80 °C under an atmosphere of CO (50 PSI) for 16 h, the reaction mixture was filtered, concentrated, diluted with EbO and extracted with EA (2 X 50 mL). The combined organic layers were dried (INfeSOb, filtered, concentrated, and purified by S1O2 chromatography (EA/ PE) to provide 900 mg (68%) of dimethyl 4- methylpyridine-2,6-dicarboxylate (INT 41-A) as a yellow solid. TLC (2: 1 EA:PE): Rf=0.20. LCMS-ESI (m/z) calculated for CioHnNC : 209.2; found 210.6 (M+H)+, tR = 0.756 min. (Method 7). NMR (400 MHz, DMSO-d6) d 8.12 (d, J = 0.7 Hz, 2H), 3.91 (s, 6H), 2.49- 2.48 (s, 3H).
References:
WO2020/198537,2020,A1 Location in patent:Page/Page column 205-206; 240-241
37645-36-6
7 suppliers
inquiry

4566-82-9
9 suppliers
inquiry