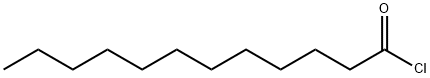
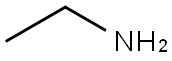
Dodecanamide, N-ethyl- synthesis
- Product Name:Dodecanamide, N-ethyl-
- CAS Number:35936-85-7
- Molecular formula:C14H29NO
- Molecular Weight:227.39
Yield:35936-85-7 77.6%
Reaction Conditions:
in dichloromethane at 0 - 20;
Steps:
4.2 Synthesis of alkamides
General procedure: Synthesis of compounds was performed using published methods (Vandevoorde et al., 2003). All solvents were purified and dried under standard methods. Organic extracts were dried using anhydrous MgSO4. NMR spectra were recorded on a Varian 400MHz (1H) and 500MHz (13C) NMR spectrometers. Chemical shifts were recorded as δ values against tetramethylsilane as an internal standard. (0026) Typical synthetic procedure: to a 2-neck dry round-bottom flask, dry CH2Cl2 (50mL was added, followed by addition of corresponding acyl chloride (1M equivalent)). The reaction vessel was cooled to 0°C followed by drop-wise addition of 10M equivalents of the corresponding amine. The reaction mixture was allowed stir at room temperature for 1h. Extraction of synthetic alkamides was performed using two successive aqueous extractions (50mL) of 5% Na2CO3, followed by 1M HCl and brine. The organic phase was dried over MgSO4. Evaporation of solvent provided a white solid product or a viscous yellow liquid depending on the fatty acyl series. High resolution mass spectra (HRMS) were obtained for each compound on a Thermo Scientific Hybrid MALDI-LTQ Orbitrap XL mass spectrometer in positive ion mode with 2,5-dihydroxybenzoic acid (DHB) as a MALDI matrix. Ions corresponding to [M+H]+ and [M+Na]+ were identified for each compound and observed masses differed from expected masses between 0.0001 and 0.0006. HRMS data for observed and calculated [M+H]+ are provided below for each compound after NMR information.
References:
Faure, Lionel;Cavazos, Ronaldo;Khan, Bibi Rafeiza;Petros, Robby A.;Koulen, Peter;Blancaflor, Elison B.;Chapman, Kent D. [Phytochemistry,2015,vol. 110,p. 58 - 71]