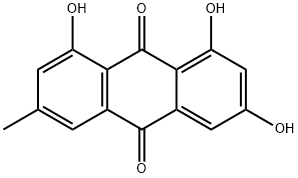
Emodin synthesis
- Product Name:Emodin
- CAS Number:518-82-1
- Molecular formula:C15H10O5
- Molecular Weight:270.24
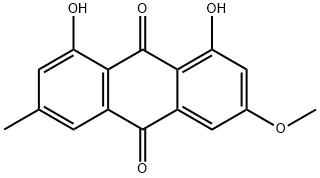
521-61-9
310 suppliers
$28.00/5mg

518-82-1
536 suppliers
$28.00/25mg
Yield:518-82-1 98%
Reaction Conditions:
with boron tribromide in dichloromethane at 0 - 20;Inert atmosphere;
Steps:
General procedure of demethylation
To a solution of starting material (1 equiv) in dichloromethane (0.1 M) was added borontribromide (3.1 equiv per one methoxy group) at 0 °C. The reaction mixture was stirred at 0 °C,and warmed to room temperature for 1 h to overnight. After that, the reaction mixture was poured into ice water. The aqueous layer was extracted with dichloromethane three times. The combined organic layer was dried over anhydrous Na2SO4, filtered and concentrated. Thecrude product was purified by column chromatography to give desired demethylated product.
References:
Chalothorn, Thidarat;Rukachaisirikul, Vatcharin;Phongpaichit, Souwalak;Pannara, Sakawrat;Tansakul, Chittreeya [Tetrahedron Letters,2019,vol. 60,# 35,art. no. 151004] Location in patent:supporting information
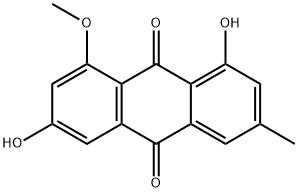
3774-64-9
28 suppliers
inquiry

518-82-1
536 suppliers
$28.00/25mg
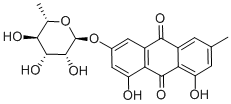
521-62-0
84 suppliers
$69.00/2mg

518-82-1
536 suppliers
$28.00/25mg
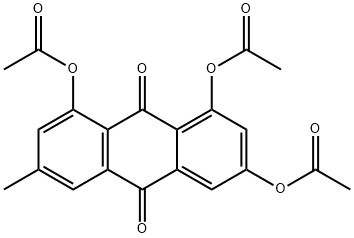
6030-60-0
18 suppliers
inquiry

518-82-1
536 suppliers
$28.00/25mg
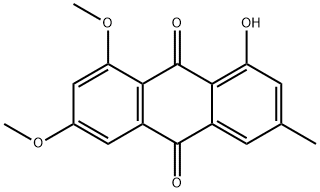
5018-84-8
1 suppliers
inquiry

518-82-1
536 suppliers
$28.00/25mg