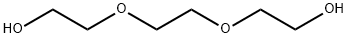Ethanol, 2-2-(2-propoxyethoxy)ethoxy- synthesis
- Product Name:Ethanol, 2-2-(2-propoxyethoxy)ethoxy-
- CAS Number:23305-64-8
- Molecular formula:C9H20O4
- Molecular Weight:192.25

106-94-5
580 suppliers
$10.00/5ml

23305-64-8
2 suppliers
inquiry
Yield:23305-64-8 18%
Reaction Conditions:
Stage #1: 2,2'-[1,2-ethanediylbis(oxy)]bisethanolwith sodium hydride in tetrahydrofuran; for 1.5 h;
Stage #2: propyl bromide in tetrahydrofuran at 20;
Steps:
8.9
Triethylene glycol (19.5 g, 0.13 mol) was dissolved in tetrahydrofuran (150 mL) and sodium hydride (2.60 g, 0.065 mol) was added portion wise over 0.5 h and the reaction was stirred for an additional 1 h. Then 1-bromopropanol (8.0 g, 0.065 mol) dissolved in tetrahydrofuran (30 mL) was added dropwise via addition funnel and the reaction was stirred overnight at room temperature. Crude reaction mixture was filtered through Celite, washed CH2Cl2, and evaporated to dryness. The resultant oil was dissolved in CH2Cl2 (250 mL), washed sat. NaCl (250 mL), H2O (250 mL), dried MgSO4, and evaporated to dryness. Column chromatography (Silica, ethyl acetate) afforded 1 a yellowish oil (2.24 g, 18% yield).
References:
US2006/18874,2006,A1 Location in patent:Page/Page column 33