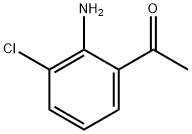
Ethanone,1-(2-amino-3-chlorophenyl)- synthesis
- Product Name:Ethanone,1-(2-amino-3-chlorophenyl)-
- CAS Number:56762-32-4
- Molecular formula:C8H8ClNO
- Molecular Weight:169.61
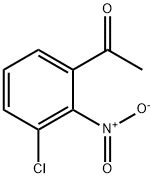
7137-38-4

56762-32-4
The general procedure for the synthesis of 2-amino-3-chlorophenyl ketone from 1-(3-chloro-2-nitrophenyl)ethanone was as follows: 1. Compound 11 (3.0 g, 0.014 mol) was dissolved in 20 mL of anhydrous CH2Cl2, SOCl2 (8.9 g, 0.069 mol) and a drop of DMF were added, and heated to reflux for 2 hours. After completion of the reaction, vacuum concentration gave compound 11a (3.1 g, yield: 95.4%) as a yellow solid, which was used directly in the next step. 2. Mg (0.4 g, 0.017 mol) was dissolved in 30 mL of anhydrous THF at 50 °C. EtOH (1.278 g, 0.028 mol) and CCI4 (0.1 mL) were added sequentially and stirred for 30 min. Subsequently, diethyl malonate (2.6 g, 0.017 mol) and EtOH (0.89 g, 0.019 mol) were added to a 10 mL solution of anhydrous THF and stirred for 2 hours at 60 °C. A 10 mL THF solution of compound 11a (3.1 g, 0.014 mol) was added and stirring was continued at 60 °C for 1 hour. After cooling, the reaction was quenched with 10 mL of 10% H2SO4 aqueous solution, and the organic layer was concentrated in vacuum to give a yellow oily material. 3. A mixture of CH3COOH (15 mL), H2SO4 (2 mL) and H2O (10 mL) was added to the above oily material and stirred at reflux for 10 hours. After completion of the reaction, it was extracted with EtOAc (50 mL x 3) and the organic layer was concentrated in vacuum to give compound 11b (2.4 g, yield: 86.5%). 4. Compound 11b (2.0 g, 10 mmol) was dissolved in 15 mL of HOAc, Fe (1.68 g, 30.0 mmol) was added, and stirred at room temperature for 4 h. After stirring, the stirring was continued at 50 °C for 8 h. The compound was extracted with 10% NaOH (50 mL x 3), and the organic layer was extracted with EtOAc (50 mL x 3). After cooling, it was adjusted to alkaline with 10% NaOH aqueous solution, extracted by EtOAc (10 mL x 3), and the organic layer was concentrated in vacuum to give compound 11d (0.998 g, yield: 59.1%). 5. To a 10 mL THF solution of compound 11d (0.8 g, 4.7 mmol) was added DMAP (0.564 g, 5.64 mmol) and CI3CCOCI (0.855 mg, 4.7 mmol) at 0 °C and stirred at room temperature for 6 hours. The reaction mixture was diluted with 50 mL of ice water, extracted with EtOAc (5 mL x 3), and the organic layer was concentrated in vacuum to give compound 11e (0.970 g, yield: 65.8%), which was used directly in the next step. 6. Compound 11e (0.9 g, 2.87 mmol) was dissolved in 5 mL of DMSO, CH3COONH4 (1.107 g, 14.4 mmol) was added and stirred at room temperature for 20 hours. The reaction mixture was diluted with cold water, the precipitate was collected by filtration and dried under vacuum to give 8-chloro-4-methyl-1H-quinazolin-2-one (0.373 g, yield: 67%). 7. 8-Chloro-4-methyl-1H-quinazolin-2-one (200 mg, 1.12 mmol) was dissolved in 15 mL of toluene, POCl3 (350 mg, 2.25 mmol) and DIPEA (270 mg, 2.25 mmol) were added, and the reaction was stirred at 120 °C for 4 hours. After completion of the reaction, it was concentrated in vacuum and the residue was washed with Et2O (25 mL x 3) to afford 2,8-dichloro-4-methyl-quinazoline (150 mg, yield: 68.2%), m/z = 197 [M + H]+.

7137-38-4
15 suppliers
inquiry

56762-32-4
38 suppliers
inquiry
Yield:56762-32-4 80%
Reaction Conditions:
with iron;acetic acid at 1; for 80 h;Cooling;Inert atmosphere;
Steps:
5 l-(2-amisio-3-chIorophenyl)ethasi-l-osie.
To a solution of l-(3-chloro-2-nitrophenyl)ethan-l-one (65 g, (0387) 0,33 mol) in glacial acetic acid (500 mL) was added iron powder (55 g, 0.98 mol). Shake the slurry until it thick. Use cooling to prevent a reflux. Let the mixture sit for 1 hr at 80C'C shaking ever hour to make sure it is mixed. Cool and quench with 10% sodium hydroxide. Extract product by shaking with ethyl acetate and then separating the emulation by centrifugation followed by decanting the organic layer. Repeat 3 times. Filter through celite, dry over sodium sulfate and evaporate in vacuo to give the aniline (44.1 g, 80%). 1H- NMR (400 MHz; CDC13); δ 7.76 id, ,/ 7.8 Hz, I H), 7.70 (d, ,/ 8.1 Hz, 1 1 1 ), 7.56 (t, J= 8.0 Hz, IH), 2.61 (s, 3H).
References:
THE SCRIPPS RESEARCH INSTITUTE;BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM;LAIRSON, Luke L.;BOLLONG, Michael J.;SCHULTZ, Peter G.;MANI, Sendurai A. WO2019/89577, 2019, A1 Location in patent:Paragraph 00167; 00166
533-17-5
162 suppliers
$7.00/5g

56762-32-4
38 suppliers
inquiry

533-17-5
162 suppliers
$7.00/5g
6953-83-9
50 suppliers
$170.00/50 mg

56762-32-4
38 suppliers
inquiry
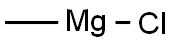
676-58-4
294 suppliers
$15.00/25ml
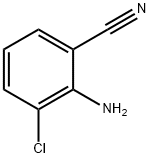
53312-77-9
161 suppliers
$7.00/100mg

56762-32-4
38 suppliers
inquiry

533-17-5
162 suppliers
$7.00/5g

6953-83-9
50 suppliers
$170.00/50 mg

56762-32-4
38 suppliers
inquiry
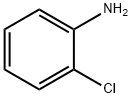
95-51-2
391 suppliers
$5.00/5 g