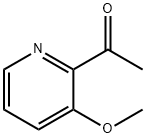
Ethanone, 1-(3-methoxy-2-pyridinyl)- (9CI) synthesis
- Product Name:Ethanone, 1-(3-methoxy-2-pyridinyl)- (9CI)
- CAS Number:379227-03-9
- Molecular formula:C8H9NO2
- Molecular Weight:151.16
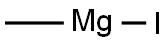
917-64-6
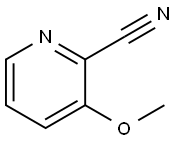
24059-89-0

379227-03-9
Under nitrogen protection, 2-cyano-3-methoxypyridine (0.88 g, 6.5 mmol) was dissolved in a three-necked flask containing 20 mL of anhydrous tetrahydrofuran. The reaction system was cooled to 0 °C, followed by slow dropwise addition of tetrahydrofuran solution of methylmagnesium iodide (3 M, 4.3 mL, 12.8 mmol). The reaction temperature was maintained at 0 °C with continuous stirring for 2 hours. Upon completion of the reaction, the reaction was quenched by the slow addition of 50 mL of water at 0 °C. The pH of the reaction solution was adjusted to 7 with 2 N hydrochloric acid and subsequently extracted with ethyl acetate (3 x 50 mL). The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. Purification by silica gel column chromatography (eluent: hexane/ethyl acetate, gradient 0-100%) afforded the target compound 1-(3-methoxypyridin-2-yl)ethanone as a colorless oil (0.79 g, yield 79%). The product was confirmed by 1H-NMR (CDCl3): δ 8.42 (dd, J = 7.7, 1.4 Hz, 1H), 7.63 (dd, J = 7.6, 1.4 Hz, 1H), 7.61 (dd, J = 7.7, 7.6 Hz, 1H), 3.82 (s, 3H), 2.14 (s, 3H). Mass spectrum (ESI+): m/z 152 ([M+H]+).
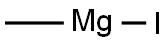
917-64-6
146 suppliers
$45.00/10ml

24059-89-0
92 suppliers
$34.00/1g

379227-03-9
67 suppliers
$43.00/100mg
Yield:379227-03-9 79%
Reaction Conditions:
in tetrahydrofuran at 0; for 2 h;Inert atmosphere;
Steps:
1-(3-Methoxy-pyridin-2-yl)ethanone
3-Methoxy-2-cyanopyridine (0.88 g, 6.5 mmol) was dissolved in anhydrous tetrahydrofuran (20 mL) in a 3-neck flask under a nitrogen atmosphere.The reaction was cooled to 0 °C, and a solution of methylmagnesium iodide in tetrahydrofuran (3M, 4.3 ml, 12.8mmol) was added. The reaction was stirred at 0 °C for 2 hours and then quenched with 50 mL of water at 0 °C. The pH was adjusted to 7 with 2N HCl, and the resulting solution was extracted with ethyl acetate (3 x 50 mL)), the combined organic layers were dried over anhydrous sodium sulfate and concentrated on a rotary evaporator to provide the crude product. Silica gel chromatography (gradient of 0 - 100% ethyl acetate in hexane) provided the product as a colorless oil (0.79g, 79% yield). 1H-NMR (CDCl3) δ 8.42 (dd, J = 7.7 and 1.4Hz, 1H), 7.63 (dd, J = 7.6 and 1.4 Hz, 1H), 7.61 (dd, J = 7.7 and 7.6 Hz, 1H),3.82 (s, 3H), 2.14 (s, 3H), (ESI+): m/z (M+H)+= 152.
References:
Lounsbury, Nicole;Mateo, George;Jones, Brielle;Papaiahgari, Srinivas;Thimmulappa, Rajash K.;Teijaro, Christiana;Gordon, John;Korzekwa, Kenneth;Ye, Min;Allaway, Graham;Abou-Gharbia, Magid;Biswal, Shyam;Childers, Wayne [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 17,p. 5352 - 5359] Location in patent:supporting information

81376-85-4
2 suppliers
inquiry

379227-03-9
67 suppliers
$43.00/100mg
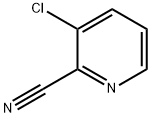
38180-46-0
245 suppliers
$6.00/1g

379227-03-9
67 suppliers
$43.00/100mg