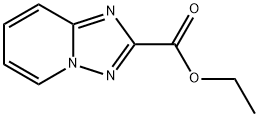
ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate synthesis
- Product Name:ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate
- CAS Number:62135-58-4
- Molecular formula:C9H9N3O2
- Molecular Weight:191.19
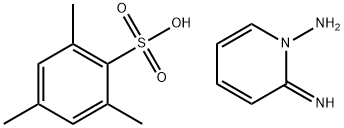
56000-35-2
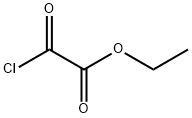
4755-77-5
![ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate](/CAS2/GIF/62135-58-4.gif)
62135-58-4
The general procedure for the synthesis of ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate from ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate using 2,4-trimethylbenzenesulfonate 1,2-diaminopyridinium (10.9 g, 35.2 mmol) and ethyl monooxynitrate of oxalyl chloride (9.62 g, 7.84 mL, 70.5 mmol) was as follows: at room temperature, ethyl monooxynitrate of oxalyl chloride was added slowly to a light red solution of 2,4-trimethyl benzenesulfonic acid 1,2-diaminopyridinium pyridine (50 mL) in a light red solution, noting that the reaction is exothermic. The color of the solution changed from light red to dark red. Subsequently, the reaction mixture was heated to 100 °C with continuous stirring overnight. After completion of the reaction, the solvent was removed by evaporation and the black residue obtained was ground with saturated aqueous sodium carbonate solution (300 mL) for 30 minutes. The reaction mixture was extracted with dichloromethane (4 x 250 mL), the organic layers were combined and dried with anhydrous magnesium sulfate. After drying, the organic phase was concentrated in vacuum to give 6.64 g of crude product. The crude product was suspended in ether (30 mL), filtered and washed with ether. The resulting precipitate was dried under vacuum to give the light brown solid product ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate (6.1 g, 31.9 mmol, 90.6% yield). Mass spectral analysis showed M = 192.2 (M + H)+.

56000-35-2
1 suppliers
inquiry

4755-77-5
343 suppliers
$9.00/25g
![ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate](/CAS2/GIF/62135-58-4.gif)
62135-58-4
37 suppliers
$45.00/10mg
Yield:62135-58-4 90.6%
Reaction Conditions:
in pyridine;diethyl ether at 100;
Steps:
64.3 Step 3:
Ethyl[1,2,4]triazolo[1,5-a]pyridine-2-carboxylate
To a light red solution of 1,2-diaminopyridinium 2,4,6-trimethylbenzenesulfonate (10.9 g, 35.2 mmol) in pyridine (50 mL) was added ethyl 2-chloro-2-oxoacetate (9.62 g, 7.84 mL, 70.5 mmol) at RT (exotherm reaction.).
The light red solution turned into a dark red solution.
The mixture was heated to 100° C. and stirred overnight.
The reaction mixture was evaporated and the black residue was triturated for 30 min with Na2CO3 (saturated aqueous solution, 300 mL).
The reaction mixture was extracted with dichloromethane (4*250 mL) and the combined organic layer was dried over MgSO4 and concentrated in vacuo to give 6.64 g of crude product.
The crude product was suspended in diethyl ether (30 mL), filtered and washed with diethyl ether.
The obtained precipitate was dried in vacuo to give the product as light brown solid (6.1 g, 31.9 mmol, 90.6%).
MS: M=192.2 (M+H)+
References:
US2013/59833,2013,A1 Location in patent:Paragraph 0515-0516
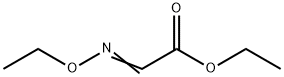
816-27-3
115 suppliers
$17.00/100mg

4931-36-6
0 suppliers
inquiry
![ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate](/CAS2/GIF/62135-58-4.gif)
62135-58-4
37 suppliers
$45.00/10mg

56000-35-2
1 suppliers
inquiry

4755-77-5
343 suppliers
$9.00/25g
![ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate](/CAS2/GIF/62135-58-4.gif)
62135-58-4
37 suppliers
$45.00/10mg