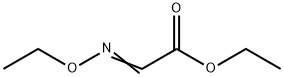
ETHOXY-IMINO-ACETIC ACID ETHYL ESTER synthesis
- Product Name:ETHOXY-IMINO-ACETIC ACID ETHYL ESTER
- CAS Number:816-27-3
- Molecular formula:C6H11NO3
- Molecular Weight:145.16

64-17-5
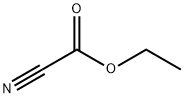
623-49-4

816-27-3
Ethyl cyanoacetate (40 g, 404 mmol) was dissolved in dichloromethane (200 mL) and cooled to 0°C under nitrogen protection. An ethanol solution of 45 wt.% hydrochloric acid (27.3 mL, 404 mmol) was added slowly and dropwise over 15 min with stirring. The reaction mixture was stirred continuously at 0 °C for 3 h, followed by standing overnight at -5 °C to -3 °C. The following day, dichloromethane (250 mL) was added to the reaction mixture and kept at 0 °C. A solution of triethylamine (113 mL, 807 mmol) in dichloromethane (50 mL) was added dropwise over 30 minutes. After the dropwise addition, the mixture was continued to be stirred at 0°C for 30 minutes. Subsequently, water (100 mL) was added and stirred for 5 minutes. The organic layer was separated, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Diethyl ether (50 mL) was added to the residue and the solid was removed by filtration. The filtrate was dried to give ethyl 2-ethoxy-2-iminoacetate as a light yellow liquid (31.0 g, 214 mmol, 52.9% yield). NMR hydrogen spectrum (400 MHz, CDCl3) δ 8.78 (s, 1H), 4.36-4.28 (m, 4H), 1.40-1.35 (m, 6H).

623-49-4
246 suppliers
$10.60/1gm:

816-27-3
115 suppliers
$17.00/100mg
Yield: 52.9%
Reaction Conditions:
Stage #1:ethyl cyanoformate with hydrogenchloride in ethanol;dichloromethane;water at -3 - 0;Inert atmosphere;
Stage #2: with triethylamine in ethanol;dichloromethane;water at 0; for 1 h;Inert atmosphere;
Steps:
4.1 Ethyl 2-ethoxy-2-iminoacetate
To a solution of ethyl carbonocyanidate (40 g, 404 mmol) in DCM (200 mL) stirred under nitrogen at 0 °C was added a solution of HC1 (45 wt. %, 27.3 mL, 404 mmol) in EtOH dropwise over 15 min. The reaction mixture was stirred at 0 °C for 3 hr and allowed to stand overnight at -5 °C to -3 °C. To the resulting mixture was added DCM (250 mL) at 0 °C. TEA (113 mL, 807 mmol) in DCM (50 mL) was added dropwise over 30 min at 0 °C. The mixture was stirred for 30 min at 0 °C, and water (100 mL) was added at 0 °C. The resulting mixture was stirred for 5 min. The organic layer was separated, dried over sodium sulfate, and evaporated. Diethyl ether (50 mL) was added to the residue and the solid was filtered. The filtrate was dried to afford ethyl 2-ethoxy-2-iminoacetate as a pale yellow liquid (31.0 g, 214 mmol, 52.9 % yield). 'HNMR (400 MHz, CDCb) d 8.78 (s, 1H), 4.36-4.28 (m, 4H), 1.40-1.35 (m, 6H).
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;ANBARI, Jill Marinis;REILLY, Michael;MAHAJAN, Mukesh K.;RATHI, Chetan WO2020/44206, 2020, A1 Location in patent:Page/Page column 82; 100

64-17-5
825 suppliers
$10.00/50g

623-49-4
246 suppliers
$10.60/1gm:

816-27-3
115 suppliers
$17.00/100mg