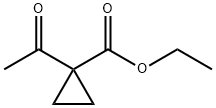
ethyl 1-acetylcyclopropanecarboxylate synthesis
- Product Name:ethyl 1-acetylcyclopropanecarboxylate
- CAS Number:32933-03-2
- Molecular formula:C8H12O3
- Molecular Weight:156.18

141-97-9
814 suppliers
$5.00/25g

106-93-4
402 suppliers
$15.00/5g

32933-03-2
116 suppliers
$41.00/250mg
Yield:32933-03-2 78.1 g (65.1%)
Reaction Conditions:
with potassium carbonate in acetone;
Steps:
R.11.1 Ethyl 1-acetylcyclopropanecarboxylate
Referential Example 11-1 Ethyl 1-acetylcyclopropanecarboxylate Ethyl acetoacetate (100 g, 0.77 mol) was dissolved in acetone (500 ml). To the thus obtained solution was added dibromoethane (361 g, 1.92 mol) and potassium carbonate (266 g, 1.92 mol) and the mixture was heated under reflux for 4 days. After filtering off insoluble matter, the filtrate was distilled under reduced pressure (80° C./8 mmHg) to obtain 78.1 g (65.1%) of the title compound as a colorless oily substance. 1 H-NMR (400 MHz, CDCl3) δ: 1.29 (3H, t, J=7.33 Hz), 1.47 (4H, s), 2.47 (3H, s), 4.21 (2H, q, J=7.33 Hz).
References:
US6121285,2000,A

141-97-9
814 suppliers
$5.00/25g

106-93-4
402 suppliers
$15.00/5g

32933-03-2
116 suppliers
$41.00/250mg

141-97-9
814 suppliers
$5.00/25g

107-06-2
437 suppliers
$14.00/25g

32933-03-2
116 suppliers
$41.00/250mg
![Sulfilimine, S-ethenyl-S-(4-methylphenyl)-N-[(4-methylphenyl)sulfonyl]-, (+)- (9CI)](/CAS/20210305/GIF/81115-90-4.gif)
81115-90-4
0 suppliers
inquiry

141-97-9
814 suppliers
$5.00/25g

32933-03-2
116 suppliers
$41.00/250mg

141-97-9
814 suppliers
$5.00/25g

106-93-4
402 suppliers
$15.00/5g

2986-03-0
0 suppliers
inquiry

32933-03-2
116 suppliers
$41.00/250mg