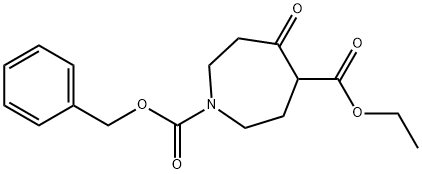
Ethyl 1-Cbz-5-oxoazepane-4-carboxylate synthesis
- Product Name:Ethyl 1-Cbz-5-oxoazepane-4-carboxylate
- CAS Number:31696-09-0
- Molecular formula:C17H21NO5
- Molecular Weight:319.35
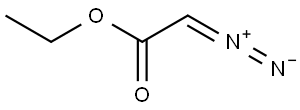
623-73-4
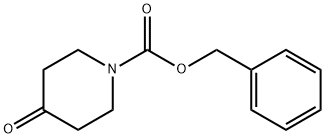
19099-93-5

31696-09-0
To a suspension of benzyl 4-oxo-1-piperazine carboxylate (11.7 g, 50.3 mmol) in ether (100 ml) at -78 °C was slowly added ethyl diazoacetate (6.84 ml, 65.3 mmol) followed by dropwise addition of boron trifluoride ether complex (6.30 ml, 50.3 mmol). After 1 hr of reaction, the reaction was gradually warmed up to room temperature to give a clarified yellow solution. Saturated aqueous potassium carbonate solution was added slowly dropwise until no gas was produced. The organic phase was separated and washed with saturated aqueous potassium carbonate solution (2 x 50 ml), dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give ethyl 1-Cbz-5-oxoazepane-4-carboxylate as a yellow oil (16.5 g, quantitative yield). The product can be used directly in the next reaction without further purification.LCMS analysis showed the following: calculated value MH+ (320); measured value 85% (MH+) m/z 320, retention time Rt=1.92 min.1H NMR (500MHz, CDCl3) δ 7.27-7.40 (5H, m), 5.05-5.19 (2H, m). 4.13-4.30 (2H, m), 3.84-3.98 (1H, m), 3.67-3.84 (2H, m), 3.34-3.57 (2H, m), 2.62-2.93 (2H, m), 1.98-2.14 (2H, m), 1.18-1.34 (3H, m).

623-73-4
151 suppliers
$26.69/5g

19099-93-5
0 suppliers
$5.00/1g

31696-09-0
63 suppliers
$34.00/250mg
Yield: 88%
Reaction Conditions:
with boron trifluoride diethyl etherate in diethyl ether
Steps:
1 Preparation of 1-benzyl 4-ethyl 5-oxo-1,4-azepanedicarboxylate
PREPARATION 1. Preparation of 1-benzyl 4-ethyl 5-oxo-1,4-azepanedicarboxylate A dry 500 ml 3-neck flask was charged with benzyl 4-oxo-1-piperidinecarboxylate (35.08 g, 150 mmol). It was dissolved in 130 ml Et2O and cooled to -45° C. Ethyldiazoacetate (20.5 ml, 195 mmol) and boron trifluoride ethyl ether (19.4 ml, 158 mmol) were added simultaneously by syringe pump over 45 minutes. The temperature was kept below -25° C. The reaction was stirred for 30 minutes longer, and then quenched with sat. NaHCO3. The ice bath was removed. The reaction was diluted with EtOAc (250 ml) and H2O (150 ml). The layers were separated, and the organic phase was dried over MgSO4. It was concentrated under reduced pressure to an orange oil. The product was purified by flash chromatography (silica gel, 40% EtOAc/hexane), yielding product as pale yellow oil (42.1 g, 88%). 1H NMR (CDCl3) δ 7.39-7.31, 5.15-5.12, 4.42-4.17, 3.96-3.83, 3.75-3.70, 3.65, 3.54-3.37, 2.08-2.03, 1.29-1.24; IR (liq.) 1743, 1702, 1476, 1455, 1443, 1425, 1371, 1318, 1295, 1238, 1213, 1178, 1098, 1068, 1028 cm-1.
References:
Pharmacia & Upjohn Company US6407092, 2002, B1

623-73-4
151 suppliers
$26.69/5g

19099-93-5
0 suppliers
$5.00/1g

31696-09-0
63 suppliers
$34.00/250mg