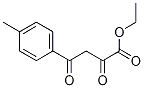
ethyl 2.4-dioxo-4-p-tolylbutanoate synthesis
- Product Name:ethyl 2.4-dioxo-4-p-tolylbutanoate
- CAS Number:5814-37-9
- Molecular formula:C13H14O4
- Molecular Weight:234.25
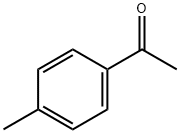
122-00-9
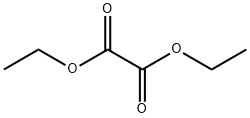
95-92-1

5814-37-9
(1) In a 250-mL three-necked flask, 24 g (0.348 mol) of sodium ethoxide and 200 mL of anhydrous toluene were added and mixed with stirring. Subsequently, 45.6 g (0.34 mol) of 4'-methylacetophenone was added slowly and dropwise over a period of 30 minutes to ensure uniform mixing. Next, 51.0 g (0.348 mol) of diethyl oxalate was added. After dropwise addition, the reaction mixture was heated to 50-60°C and stirred continuously for 4 hours. Upon completion of the reaction, it was cooled to room temperature, washed with 5% dilute hydrochloric acid solution and the pH was adjusted to the appropriate value. Liquid-liquid separation was carried out and the toluene layer was collected and dried with anhydrous sodium sulfate for 30 minutes. After filtration, the filtrate was distilled under reduced pressure to recover toluene. The residue was recrystallized from 95% ethanol to give 73.3 g of Intermediate 1 (purity: 99.1%) in 92% yield.
Yield:5814-37-9 92%
Reaction Conditions:
with potassium etoxide in toluene at 50 - 60; for 4 h;
Steps:
11.1; 12.1; 13.1; 14.1 Example 11, Synthesis of 5-(4-methylbenzoyl)-2-hydroxypyrimidine
(1) In a 250 ml three-necked flask,Add 24 g (0.348 mol) of sodium ethoxide and 200 mL of dry toluene.Stir,Add dropwise (30 minutes dropwise) 45.6g (0.34mol)Uniform mixing of 4-methylacetophenone and 51.0 g (0.348 mol) of diethyl oxalateThe solution is heated to 50 to 60 ° C after the dropwise addition.The reaction was stirred for 4 h.Cool to room temperature,Wash with 5% dilute hydrochloric acid solution,And adjust the PH value to 5 to 6,Liquid separation,Collecting the toluene layer,Dry with anhydrous sodium sulfate for 30 minutes.filter,The filtrate pump is distilled under reduced pressure to recover toluene.The residue was recrystallized from 95% ethanol to give Intermediate 1 (purity: 99.1%) 73.3 g.The yield was 92%.
References:
CN108148003,2018,A Location in patent:Paragraph 0144; 0145; 0150; 0151; 0156; 0157; 0162; 0163
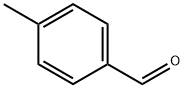
104-87-0
516 suppliers
$6.00/25g

5814-37-9
43 suppliers
$10.00/250mg
536-50-5
123 suppliers
$29.00/1g

5814-37-9
43 suppliers
$10.00/250mg