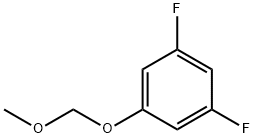
ethyl 2,6-difluoro-3-(MethoxyMethoxy)benzoate synthesis
- Product Name:ethyl 2,6-difluoro-3-(MethoxyMethoxy)benzoate
- CAS Number:1384476-81-6
- Molecular formula:C11H12F2O4
- Molecular Weight:246.21
Yield:1384476-81-6 82%
Reaction Conditions:
Stage #1: 1,3-difluoro-5-(methoxymethoxy)benzenewith sec.-butyllithium in tetrahydrofuran;hexane at -78; for 2 h;Inert atmosphere;
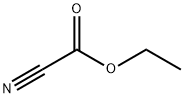
Stage #2: ethyl cyanoformate in tetrahydrofuran;hexane at -40; for 1 h;
Steps:
1 Example 1 ethyl 2,6-difluoro-3- (methoxymethoxy) benzoate (D)
Compound C (4.70g, 27.0mmol) was dissolved in anhydrous THF (108ml),N2 protection, dropwise addition of sec-butyl lithium 1.32M n-hexane solution (24.5ml, 32.4mmol) at -78 ° C, stirring reaction was continued for 2h, and ethyl cyanoformate (4.09ml,40.5 mmol), control the temperature not to exceed -40 ° C, continue to stir the reaction for 1 h, quench the reaction with water (100 ml), extract with ethyl acetate (2 × 100 ml), combine the organic layers, wash with saturated brine and dry over anhydrous magnesium sulfate Concentrate to dryness to a pale yellow oil, columnChromatography (petroleum ether / ethyl acetate = 10: 1) gave 5.45 g of colorless oil with a yield of 82.0%;
References:
CN103724360,2016,B Location in patent:Paragraph 0138-0139