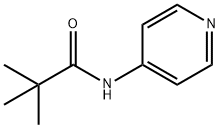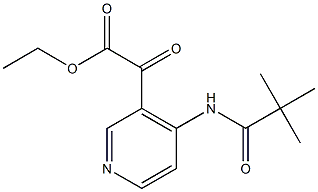
Ethyl 2-oxo-2-(4-pivalamidopyridin-3-yl)acetate synthesis
- Product Name:Ethyl 2-oxo-2-(4-pivalamidopyridin-3-yl)acetate
- CAS Number:191338-94-0
- Molecular formula:C14H18N2O4
- Molecular Weight:278.3037
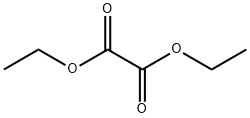
95-92-1
439 suppliers
$5.00/5G

191338-94-0
5 suppliers
inquiry
Yield:191338-94-0 52%
Reaction Conditions:
Stage #1: 4-N-pivaloylaminopyridinewith n-butyllithium in tetrahydrofuran;hexane at -78 - 0; for 3 h;
Stage #2: oxalic acid diethyl ester in tetrahydrofuran;hexane at -78 - 20; for 0.75 h;
Steps:
2.2A
Preparation of (2,2-Dimethyl-propionylamino)-pyridinyl - oxo-acetic acid ethyl esters 2A: [4- (2, 2-Dimethyl-propionylamino)-pyridin-3-yl]-oxo-acetic acid ethyl ester [084] Following procedures generally taught in J. A. Turner, J. Org. Chemin. 1983, 48, 3401-8; and C. Rivalle & E. Bisagni, J. Heterocyclic Chem., 1997, 34, 441-4, a 3-necked, 500 mL round bottom flask with thermometer and addition funnel were flame-dried under N2.2, 2- Dimethyl-N-pyridin-3-yl-propionamide (4.46 g, 25.0 mmol) was added, followed by THF (50 mL). The solution was cooled to-78. °C (to control the pending exotherm), and n-BuLi (39 mL of a 1.6 M solution in hexanes, 2.5 equiv) was added dropwise via addition funnel with vigorous stirring of the slurry, keeping internal temp below-50 °C during addition. Once the 77-BuLi addition was complete (the solution was yellow and homogeneous), the mixture was warmed to 0 °C for 3 h (a white precipitate emerges). The solution was cooled back down to - 78 °C and diethyl oxalate (8.84 mL, 2.6 equiv) in THF (13 mL) was added dropwise via syringe (mild exotherm observed). Once the addition was complete, the reaction was stirred 15 min at-78 °C, then warmed to room temperature over 15 min and stirred an additional 15 min (a dark red-orange solution emerges as the solution was stirred at room temperature). The mixture was poured onto ice and extracted with Et20, washing the extract once with water. The solution was dried (MgSO4), filtered, and concentrated to a dark orange oil. Purification by biotage (50% EtOAc/hexanes) afforded the product as an orange oil (3.61 g, 52%). Rf (prod) = 0.57 (50% EtOAc/hexanes). lH NMR (400 MHz, DMSO-d6) 8 10.69 (s, 1 H), 8.79 (s, 1 H), 8. 66 (d, J= 6.4 Hz, 1 H), 8.00 (d, J= 5.6 Hz, 1 H), 4.32 (q, J= 7.0 Hz, 2 H), 1.28 (t, J= 7.0 Hz, 3 H), 1.21 (s, 9 H). MS (LR-APCI) calcd. for Cl4HlgN204 (M+H) 279.1 ; found 279.1.
References:
WO2005/56552,2005,A1 Location in patent:Page/Page column 22-23
3282-30-2
380 suppliers
$19.85/100ml

191338-94-0
5 suppliers
inquiry
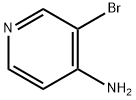
13534-98-0
284 suppliers
$6.00/250mg

191338-94-0
5 suppliers
inquiry
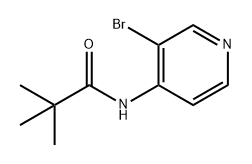
239137-59-8
2 suppliers
inquiry

191338-94-0
5 suppliers
inquiry