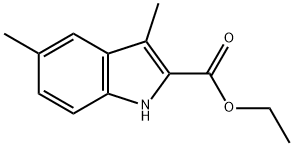
ethyl 3,5-dimethyl-1H-indole-2-carboxylate synthesis
- Product Name:ethyl 3,5-dimethyl-1H-indole-2-carboxylate
- CAS Number:16423-76-0
- Molecular formula:C13H15NO2
- Molecular Weight:217.26
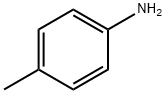
106-49-0
415 suppliers
$5.00/5G
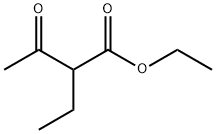
607-97-6
216 suppliers
$10.00/1g

16423-76-0
17 suppliers
$289.00/500mg
Yield:16423-76-0 51%
Reaction Conditions:
Stage #1: p-toluidinewith hydrogenchloride;sodium acetate;sodium nitrite in water at -5 - 0; pH=3.4; for 0.25 h;
Stage #2: ethyl 2-ethyl-3-oxobutanoatewith potassium hydroxide in ethanol;water at 0; pH=5 - 6; for 15 h;
Stage #3: with hydrogenchloride in ethanol; for 2 h;Heating / reflux;
Steps:
[0048] Ethyl 3,5-dimethylindole-2-carboxylate(7). To a solution of 5.6 g (0.052 mol) of p-toluidine in 15 mL of conc. HCl and 25 mL of H2O, was added dropwise a solution of 3.9 g (0.057 mol) of NaNO2 in 5 mL of H2O at -5° C. After complete addition, the mixture was stirred at 0° C. for 15 min and brought to pH 374 by addition of 5 g of sodium acetate. In a separate flask, a solution of 9 g (0.055 mol) of ethyl α-ethylacetoacetate in 40 mL EtOH was cooled to 0° C. and combined with 3.5 g KOH (0.064 mol) in 10 ml H2O. To this solution was added 70 g ice followed by addition of the diazonium salt prepared above. The mixture was then adjusted to pH 5-6 and stirred at 0° C. for 15 h. The completed reaction was extracted 5× with 50 mL portions of CH2Cl2 and the combined extracts were washed with brine and dried over Na2SO4. Most of the solvent was removed under reduced pressure, and the liquid residue was added dropwise to a solution of 14.5% ethanolic HCl at reflux. After refluxing this mixture for 2 h, the solvent was removed under reduced pressure and the residue was combined with a mixture of 50 mL of water and 100 mL of CH2Cl2. The CH2Cl2 layer was removed and the aqueous layer was extracted 3× with 50 mL portions of CH2Cl2. The combined extracts were dried over Na2SO4 and concentrated to a residue, which was applied to a silica gel column prepared with CH2Cl2. Product fractions were evaporated to afford a white solid: 5.74 g(51% )yield; mp 131-133° C.; TLC (CHCl3) Rf=0.25; IR (KBr pellet) 3306, 2924, 2854, 1680, 1548, 1475, 1384, 1332, 1263, 798 cm-1; 1HNMR (CDCl3) δ8.56 (1H, bs, indole proton), 7.43 (1H, s, 4-proton), 7.26 (1H, d, J=8.4 Hz, 7-proton), 7.15 (1H, d, J=8.4 Hz, 6-proton), 4.40 (2H, q, J=7.2 Hz, methylene) 2.59 and 2.46 (6H, 2s, 3,5-dimethyl), 1.42 (3H, t, J=7.2 Hz, methyl of ethyl); MS [EI mode] m/z 217(M+), 188 (M+-CH2CH3), 171, 142, 115. Anal. Calcd (C13H15NO2) C, H, N.
References:
US2004/6054,2004,A1 Location in patent:Page/Page column 12

106-49-0
415 suppliers
$5.00/5G

16423-76-0
17 suppliers
$289.00/500mg
![2-Butenoic acid, 3-[2-[(1,1-dimethylethoxy)carbonyl]diazenyl]-, ethyl ester](/CAS/20210111/GIF/95239-03-5.gif)
95239-03-5
0 suppliers
inquiry

16423-76-0
17 suppliers
$289.00/500mg
15933-07-0
123 suppliers
$8.00/1g

621-94-3
0 suppliers
inquiry

16423-76-0
17 suppliers
$289.00/500mg