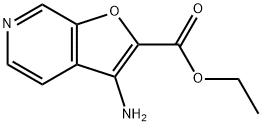
Ethyl 3-aminofuro[2,3-c]pyridine-2-carboxylate synthesis
- Product Name:Ethyl 3-aminofuro[2,3-c]pyridine-2-carboxylate
- CAS Number:927804-72-6
- Molecular formula:C10H10N2O3
- Molecular Weight:206.2
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethyl 3-aminofuro[2,3-c]pyridine-2-carboxylate](/CAS2/GIF/927804-72-6.gif)
927804-72-6
16 suppliers
$60.00/10mg
Yield: 66%
Reaction Conditions:
Stage #1:ethyl 3-(tert-butoxycarbonylamino)furo[2,3-c]pyridine-2-carboxylate with trifluoroacetic acid in dichloromethane at 0 - 20;
Stage #2: with hydrogenchloride;water in dichloromethane
Stage #3: with sodium hydroxide;sodium hydrogencarbonate in water;ethyl acetate; pH=~ 7Product distribution / selectivity;
Steps:
65.B
Step B: Ethyl 3-aminofuro[2,3-c]pyridine-2-carboxylate: The crude product from step A was dissolved in cold (0° C.) dichloromethane (40 mL) and to this was added TFA (40 mL) dropwise via an addition funnel. The cold bath was removed and the reaction mixture was left at ambient temperature overnight. The reaction mixture was concentrated and the residue was dissolved in 2N HCl (200 mL). The aqueous layer was washed with ethyl acetate. The acidic aqueous layer was transferred to a 2 L Erlenmeyer flask containing 200 mL 2N NaOH and 400 mL ethyl acetate. Solid NaHCO3 was carefully added in small portions until pH7. The aqueous layer was extracted with ethyl acetate. The combined organics were dried, filtered and concentrated to give the product as a solid. (3.0 g, 66%). 1HNMR (400 MHz, CDCl3) δ 8.9 (s, 1H), 8.5 (d, J=5.4 Hz, 1H), 7.5 (d, J=5.5 Hz, 1H), 5.0 (bs, 2H), 4.5 (q, J=7.0 Hz, 2H), 1.5 (t, J=7.1 Hz, 3H). MS (APCI) m/z 307.2 (M+1).
References:
Miknis, Greg;Lyssikatos, Joseph P.;Laird, Ellen;Tarlton, Eugene;Buckmelter, Alexandre J.;Ren, Li;Rast, Bryson;Schlacter, Stephen T.;Wenglowsky, Steven Mark US2007/49603, 2007, A1 Location in patent:Page/Page column 60
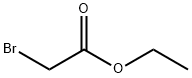
105-36-2
349 suppliers
$5.00/5 g
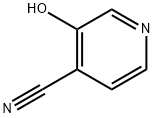
87032-82-4
42 suppliers
inquiry
![Ethyl 3-aminofuro[2,3-c]pyridine-2-carboxylate](/CAS2/GIF/927804-72-6.gif)
927804-72-6
16 suppliers
$60.00/10mg
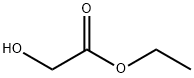
623-50-7
280 suppliers
$5.00/1g
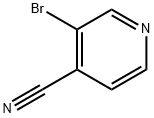
13958-98-0
163 suppliers
$10.00/100mg
![Ethyl 3-aminofuro[2,3-c]pyridine-2-carboxylate](/CAS2/GIF/927804-72-6.gif)
927804-72-6
16 suppliers
$60.00/10mg