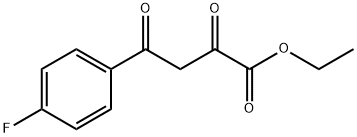
ethyl 4-(4-fluorophenyl)-2,4-dioxobutanoate synthesis
- Product Name:ethyl 4-(4-fluorophenyl)-2,4-dioxobutanoate
- CAS Number:31686-94-9
- Molecular formula:C12H11FO4
- Molecular Weight:238.21
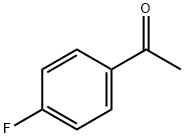
403-42-9
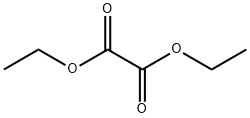
95-92-1

31686-94-9
Synthesis of ethyl 4-(4-fluorophenyl)-2,4-dioxobutanoate: In a 2L round-bottomed flask fitted with a reflux condenser tube and a CaCl2 drying tube, sodium metal (49.9 g, 2.17 mol, 3.0 eq.) was slowly dissolved in ethanol (1.0 L). After the resulting sodium ethanol solution was cooled to about 10°C, a mixture of 4-fluoroacetophenone (100.0 g, 0.72 mol) and diethyl oxalate (295 mL, 2.17 mol, 3.0 eq.) was slowly added. The reaction mixture was stirred for about 15 minutes and then placed in a refrigerator to stand overnight. Subsequently, the reaction mixture was poured into a mixture consisting of concentrated hydrochloric acid solution (300 mL) and ice (about 1 kg). The resulting precipitate was collected by filtration and washed with plenty of water and finally dried in a vacuum dryer using P2O5/KOH. The product was a light yellow powder, yield: 165.5 g (96% yield).

403-42-9
553 suppliers
$5.00/10g

95-92-1
439 suppliers
$5.00/5 g

31686-94-9
57 suppliers
$18.00/100mg
Yield:31686-94-9 96%
Reaction Conditions:
with ethanol;sodium at 10;
Steps:
Synthesis of ethyl 4-(4-fluorophenyl)-2,4-dioxobutanoate
Synthesis of ethyl 4-(4-fluorophenyl)-2,4-dioxobutanoate:In a 2L round bottom flask equipped with a reflux condenser and a CaCl2 drying tube metallic sodium (49.9 g, 2.17 mol, 3.0 equiv.) was dissolved in ethanol (1.0 L) carefully. The resulting sodium ethylate solution was cooled to about 10 °C then the mixture of l -(4-fluorophenyl)ethan-l-one (100.0 g, 0.72 mol) and diethyl oxalate (2) (295 mL, 2.17 mol, 3.0 equiv.) was poured slowly into it. The reaction mixture was stirred for about 15 minutes, and then was allowed to stand in a refrigerator overnight. The reaction mixture was poured into a mixture of cone. HC1 solution (300 mL) and ice (c.a. 1 kg). The resulting precipitate was filtered off and washed with plenty of water, finally dried in a vacuum desiccator over P2O5/KOH. Yield: 165.5 g (3) (96%) as a light yellow .powder.
References:
THE ROCKEFELLER UNIVERSITY;AHN, Hyung Jin;GLICKMAN, J. Fraser;STRICKLAND, Sidney WO2015/95337, 2015, A2 Location in patent:Paragraph 0030