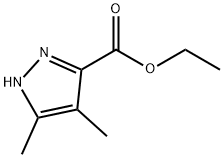
ethyl 4,5-diMethyl-1H-pyrazole-3-carboxylate synthesis
- Product Name:ethyl 4,5-diMethyl-1H-pyrazole-3-carboxylate
- CAS Number:15803-27-7
- Molecular formula:C8H12N2O2
- Molecular Weight:168.19
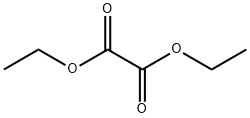
95-92-1
439 suppliers
$5.00/5 g
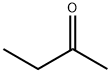
78-93-3
0 suppliers
$30.80/500ml

15803-27-7
47 suppliers
$80.00/100mg
Yield: 4.6 g
Reaction Conditions:
Stage #1:oxalic acid diethyl ester;butanone with pyrrolidine in ethanol at 20; for 13 h;Reflux;
Stage #2: with hydrazine hydrate in ethanol at -5; for 2.5 h;Reagent/catalyst;
Steps:
1-3 Example 1
Using diethyl oxalate and hydrazine hydrate as raw materials, 3,4-dimethylpyrazole-5-ethyl carboxylate compound I-1 was prepared.In a 100mL three-necked flask equipped with a thermometer, magnetic stirring and constant pressure dropping funnel, add 0.21g (3mmol) pyrroline Cat-1 and 50mL ethanol, and drop into the constant pressure dropping funnel within 1.0 hour at room temperature. A mixed solution of 2.16g (30mmol) 2-butanone IV and 4.6g (32mol) diethyl oxalate III-1. After the dripping is completed, the reaction solution is heated to a reflux state, and the reaction is continued for 12h. Remove volatile components, extract with ethyl acetate (50mL×2), wash twice with water, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the crude product of ethyl 3-methylacetylacetonate II-1, which is directly without purification. Used in the next step of synthesis.In a 100mL three-necked flask equipped with a thermometer, a magnetic stirring and a constant pressure dropping funnel, 5.16g (30mmol) of the crude product of ethyl 3-methylacetylacetonate II-1 was dissolved in 50mL of ethanol at a temperature of -5°C In 1.5h, 2.35g (37.5mmol) of 80% hydrazine hydrate V-1 was added dropwise. After the dropwise addition was completed, the reaction was kept for 1 hour. Solvent under reduced pressure, extract with ethyl acetate (250mL×2), wash twice with water, dry with anhydrous sodium sulfate, remove the solvent, and purify the residue by distillation under reduced pressure to obtain 4.60g of 3,4-dimethylpyrazole-5- Methyl formate, the yield is 91%
References:
CN112824393, 2021, A Location in patent:Paragraph 0046-0060