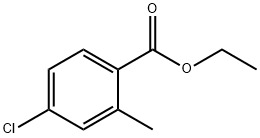
Ethyl 4-chloro-2-methylbenzoate synthesis
- Product Name:Ethyl 4-chloro-2-methylbenzoate
- CAS Number:15393-58-5
- Molecular formula:C10H11ClO2
- Molecular Weight:198.65
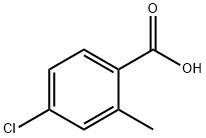
7499-07-2
194 suppliers
$6.00/1g
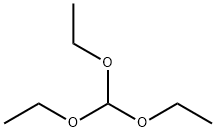
122-51-0
515 suppliers
$10.00/5ml

15393-58-5
26 suppliers
$19.00/5g
Yield:15393-58-5 95%
Reaction Conditions:
with ethanol;sulfuric acid
Steps:
11
A sample of 4-chloro-2-methylbenzoic acid was converted to the ethyl ester using ethanol/triethyl orthoformate/sulfuric acid in >95% yield. Reduction of the ester with LiAlH4/tetrahydrofuran gave 4-chloro-2-methylbenzyl alcohol in 97% yield as a colorless oil. Subsequent treatment with thionyl chloride/pyridine/CH2Cl2 gave the substituted benzyl chloride. The latter, on treatment with 40% excess sodium cyanide in DMF at 90° C. for 20 min. and normal work-up and crystallization from hexane/butyl chloride (2 crops) gave 4-chloro-2-methylbenzyl cyanide in 80% overall yield.This latter compound on treatment with formaldehyde in the presence of DBU was found to quickly form an equilibrium at relatively low conversion to the desired alcohol which was then slowly dehydrated to an atroponitrile. Consequently, a mixture of the nitrile (688 mg, 4.15 mmol) and formaldehyde in THF (2.75 mL) was treated with DBU (55 μL), then quenched at 7 min with 3N HCl. Work-up recovered starting material (528 mg) by crystallization from butyl chloride/hexane. Two cycles of retreatment followed by flash chromatography (5% 90% ethyl acetate in hexane) recovered 313 mg of the desired alcohol as a pale yellow oil. TLC: Rf 0.13 (silica gel, ethyl acetate:hexane=1:4)
References:
US2008/153905,2008,A1 Location in patent:Page/Page column 4

7499-07-2
194 suppliers
$6.00/1g

15393-58-5
26 suppliers
$19.00/5g
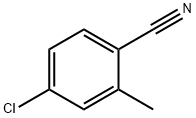
50712-68-0
149 suppliers
$7.00/1g

15393-58-5
26 suppliers
$19.00/5g
120-66-1
159 suppliers
$10.00/5g

15393-58-5
26 suppliers
$19.00/5g
