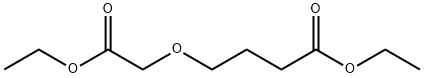
ethyl 4-((ethoxycarbonyl)methoxy)butanoate synthesis
- Product Name:ethyl 4-((ethoxycarbonyl)methoxy)butanoate
- CAS Number:388109-23-7
- Molecular formula:C10H18O5
- Molecular Weight:218.25
Yield:388109-23-7 87%
Reaction Conditions:
dirhodium tetraacetate in dichloromethane at 20; for 73.5 h;
Steps:
1.1
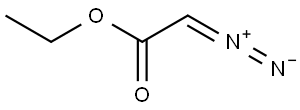
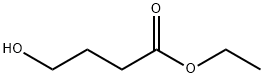
Example 1; Λ/-((3S,4S)-3-r4-(5-cvano-2-thienyl)phenyl1tetrahvdro-2/-/-pyran-4-yl)propane-2-sulfonamideStep 1 : Preparation of ethyl 4-(2-ethoxy-2-oxoethoxy)butanoate. A flask was charged with ethyl 4-hydroxybutanoate (H. Smith and R. Jones, Synthetic Communications, 1994, 24, 2743-2747) (19.08 g, 100 mMol), rhodium(ll) acetate (445 mg, 1.01 mMol) and methylene chloride (400 ml_). Ethyl diazoacetate (18.1 ml_, 152 mMol) in methylene chloride (100 ml_) was added drop-wise via an addition funnel over a period of 1.5 hours and the reaction was stirred at room temperature for 72 hours. The reaction mixture was filtered through a plug of silica gel, the plug was washed with methylene chloride, and the filtrate was concentrated to a light yellow oil. The crude oil was purified by fractional distillation at reduced pressure. The fraction boiling between 70-80°C at ~1 torr was collected to give ethyl 4-(2-ethoxy-2- oxoethoxy)butanoate as a clear oil. Yield: 19.20 g, 87 mMol, 87%. 1H NMR (400 MHz, CHLOROFORM-c/) δ 1 .22 - 1 .29 (m, 6 H), 1 .89 - 1 .97 (m, 2 H), 2.43 (t, 2 H), 3.56 (t, 2 H), 4.04 (s, 2 H), 4.12 (q, 2 H), 4.20 (q, 2 H).
References:
WO2010/38167,2010,A1 Location in patent:Page/Page column 39-40