ethyl (4-formyl-2-iodo-6-methoxyphenoxy)acetate synthesis
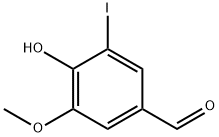
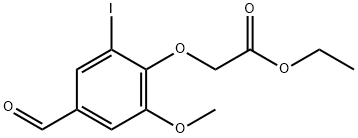
- Product Name:ethyl (4-formyl-2-iodo-6-methoxyphenoxy)acetate
- CAS Number:428490-71-5
- Molecular formula:C12H13IO5
- Molecular Weight:364.13
Yield:428490-71-5 49%
Reaction Conditions:
with caesium carbonate in acetone; for 1 h;Reflux;
Steps:
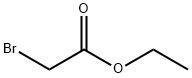
Ethyl 2-(2-iodo-4-formyl-6-methoxyphenoxy)acetate: To a solution of 5-iodovanilline (502 mg, 1.8 mmol) in acetone (25 mL) was added cesium carbonate (766 mg, 2.35 mmol), followed by ethyl bromoacetate (0.30 mL, 2.7 mmol). The mixture was refluxed in acetone under stirring for 1 hour. After evaporation of acetone under reduced pressure, 100 mL of water were added and the mixture was extracted with dichloromethane (200 mL). The organic phase was washed with brine (100 mL), dried over magnesium sulfate and concentrated under vacuum. The residue was purified by column chromatography over silica gel (eluent: dichloromethane) to give 320 mg of a yellow solid (49% yield). 1H NMR (250 MHz, CD3COCD3): δ 1.28 (t, J = 7.2 Hz, 3H), 3.97 (s, 3H), 4.23 (q, J = 7.2 Hz, 2H), 4.85 (s, 2H), 7.55 (s, 1H), 7.95 (s, 1H), 9.89 (s, 1H). 13C NMR (126 MHz, CD3COCD3): δ 15.2, 57.4, 62.1, 70.5, 92.4, 113.9, 135.1, 135.5, 153.1 (2C), 169.4, 169.5, 191.0.
References:
WO2012/156931,2012,A1 Location in patent:Page/Page column 12-13