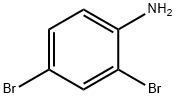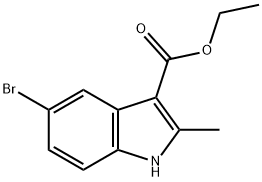
ethyl 5-bromo-2-methyl-1H-indole-3-carboxylate synthesis
- Product Name:ethyl 5-bromo-2-methyl-1H-indole-3-carboxylate
- CAS Number:1245933-87-2
- Molecular formula:C12H12BrNO2
- Molecular Weight:282.13
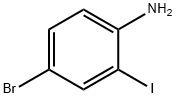
66416-72-6
192 suppliers
$6.00/250mg

141-97-9
814 suppliers
$5.00/25g

1245933-87-2
14 suppliers
inquiry
Yield:1245933-87-2 90%
Reaction Conditions:
with copper(l) iodide;caesium carbonate;1,1'-bi-2-naphthol in dimethyl sulfoxide at 50;
Steps:
12 Scheme 13.5
To a mixture of 4-Bromo-2-iodoaniline (1.38 g, 4.6 mmol) ethyl acetoacetate (0.67 g, 5.1 mmol), Cul (0.1 g, 0.52 mmol), and BINOL (0.2 g, 0.70 mmol) was added Cs2C03 (1.5 g, 4.60 mmol) in DMSO. After addition, the reaction mixture was heated at 50°C for overnight. After reaction was completed, the reaction mixture was diluted with Sat. NH4CI and EtOAc. The organic layer was washed with brine and dried over MgS04 to afford Compound 106 (ethyl 5-bromo-2-methyl-lH-indole-3-carboxylate) (1.17 g, 4.15 mmol, yield 90%). The product was used in next step without further purification.
References:
WO2019/108943,2019,A1 Location in patent:Paragraph 0280; 0322; 0323
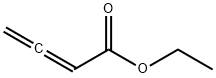
14369-81-4
68 suppliers
$60.00/100mg

66416-72-6
192 suppliers
$6.00/250mg

1245933-87-2
14 suppliers
inquiry