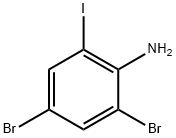
4-BROMO-2-IODOANILINE synthesis
- Product Name:4-BROMO-2-IODOANILINE
- CAS Number:66416-72-6
- Molecular formula:C6H5BrIN
- Molecular Weight:297.92
106-40-1

66416-72-6
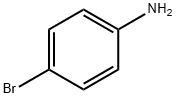
General procedure: Iodination of aryl amines was carried out under solvent-free conditions. DBDABCODCI (0.5 mmol) was co-milled with 4-bromoaniline (1 mmol) in a porcelain mortar at room temperature. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, ethyl acetate was added to the mixture and subsequently filtered. The organic layer was washed with 5% aqueous sodium thiosulfate and dried with anhydrous magnesium sulfate. The solvent was removed under reduced pressure and the resulting crude product was purified by column chromatography, the eluent being a solvent mixture of ethyl acetate and hexane. The purified product was characterized by melting point determination and 1H NMR spectroscopy.
615-43-0
428 suppliers
$5.00/5g

66416-72-6
200 suppliers
$6.00/250mg
Yield:66416-72-6 92%
Reaction Conditions:
with ammonium molybdate;sodium perborate;acetic acid;potassium bromide at 0 - 20; for 3 h;
Steps:
44.a EXAMPLE 44; Step a) N- (5-BROMO-4'-FLUORO-1, 1'-BIPHENYL-2-YL) PROPANAMIDE
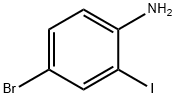
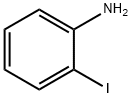
A vigorously stirred suspension of 2-iodoaniline (10. 0 g, 45.7 MMOL), potassium bromide (6.52 g, 54.8 MMOL), and ammonium molybdat tetrahydrate (0.57 g, 0.46 MMOL) in acetic acid (54 mL) was cooled to 0°C, treated with sodium perborate (7.74 g, 50.3 MMOL), and stirred for two hours as the reaction warmed to room temperature. After adding water and stirring one additional hour, the reaction mixture was poured into ice-water and filtered. The filtered solid was washed with water, dissolved in ethyl acetate (250 mL), and washed with a saturated, aqueous, potassium carbonate solution (2 x 75 mL). The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford the intermediate, 4 bromo-2- iodoaniline, as a red solid (12.56 g, 92%). [0196] 5-Bromo-4'-fluoro-1, 1'-biphenyl-2-ylamine was prepared from the above intermediate, 4-bromo-2-iodoaniline (12.6 g, 42.2 MMOL), 4-FLUOROPHENYLBORONIC acid (5.9 g, 42 MMOL), [1,1'-bis (diphenylphosphino) ferrocene] DICHLOROPALLADIUM (II) COMPLEX with DICHLOROMETHANE (0.69 g, 0.84 MMOL), and a 5 N aqueous sodium hydroxide solution (85 mL, 425 MMOL) according to the procedure and in the same manner as described in Example 32, Method A, Step a. [0197] The title compound was prepared from the above 5-bromo-4'-fluoro-1, 1'- biphenyl-2-ylamine (10.1 g, 37.8 MMOL), propionic anhydride (5.33 mL, 41.6 MMOL), 4- (N, N-dimethylamino) pyridine (0.46 g, 3.8 MMOL), and pyridine (12.3 mL, 151 MMOL), according to the same procedure, to yield N- (5-BROMO-4'-FLUORO-1, 1'-BIPHENYL-2- YL) propanamide as a solid (7.74 g, 64% from 4-bromo-2-iodoaniline), m. p. 133°C ; MS [(-ESI), M/Z] : 320 [M-H]- ; 'H NMR (500 MHz, DMSO-d6) S : 9.21 (s, 1 H), 7.52 (dd, J = 2.4, 8.5 Hz, 1 H), 7.47 (d, J = 2.2 Hz, 1H), 7.43-7. 38 (m, 3H), 7.28-7. 23 (m, 2H), 2.13 (q, J = 7.5 Hz, 2H), 0.95 (t, J = 7. 5 HZ, 3H); Anal. CALCD for C15H13BRFNO : C, 55.92 ; H, 4.07 ; N, 4.35. Found: C, 55.79 ; H, 4.08 ; N, 4.31
References:
WO2004/50631,2004,A1 Location in patent:Page 116

106-40-1
483 suppliers
$12.00/5g

66416-72-6
200 suppliers
$6.00/250mg

106-40-1
483 suppliers
$12.00/5g

66416-72-6
200 suppliers
$6.00/250mg
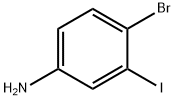
63037-64-9
46 suppliers
inquiry

615-43-0
428 suppliers
$5.00/5g

66416-72-6
200 suppliers
$6.00/250mg
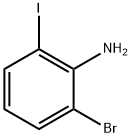
84483-27-2
56 suppliers
$38.00/100mg