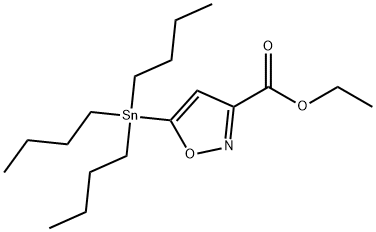
ETHYL 5-TRIBUTYLSTANNANYLISOXAZOLE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 5-TRIBUTYLSTANNANYLISOXAZOLE-3-CARBOXYLATE
- CAS Number:126085-91-4
- Molecular formula:C18H33NO3Sn
- Molecular Weight:430.17
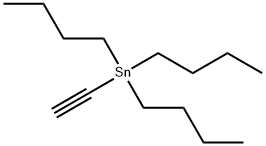
994-89-8
144 suppliers
$30.00/1g
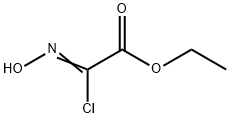
14337-43-0
206 suppliers
$9.00/1g

126085-91-4
54 suppliers
$240.00/500mg
Yield: 78%
Reaction Conditions:
with triethylamine in diethyl ether at 20;Cooling;Inert atmosphere;
Steps:
2.2-A
Example 2; 1 _(4-(5 -(5 -(Pyridin-2-yl)-4-(trifruorometriyl)isoxazol-3 -yl)- 1 ,2,4-oxadiazol-3 - yl)benzyl)azetidine-3 -carboxylic acid; 2-A. 5-(Tributylstannyl)isoxazole-3-carboxylate; O-NBu OEt (2-A)[00126] An oven dried 500 mL round bottom flask equipped with a stir bar was cooled under a stream of dry nitrogen. Ethyl chlorooximidoacetate (4.95 g, 32.7 mmol), diethyl ether (100 mL), and tributyl(ethynyl)tin (9.45 mL, 32.7 mmol) were added to give a clear pale yellow solution. Triethylamine (6.83 mL, 49.0 mmol) was added dropwise via syringe. After -500 μL had been added, the solution became cloudy. After the addition of ~2 mL, the solution began to boil, so a cold water bath was introduced and the rate of addition was slowed. The triethylamine was added slowly over 10 minutes to give a pale yellow suspension which was stirred at room temperature overnight. The solution was cooled using a dry-ice bath, filtered cold, and rinsed with cold diethyl ether. The filtrate was evaporated and placed under high vacuum to afford a pale, amber oil (15g). The oil was purified by flash chromatography (ISCO, 33Og silica gel, eluting with 5% to 20% ethyl acetate in hexanes). The product fractions were evaporated and placed under high vacuum to afford ethyl 5-(tributylstannyl)isoxazole-3-carboxylate (11 g, 25.3 mmol, 78% yield) as a clear colorless oil. The product had an HPLC retention. Time = 4.47 min. - Column: YMC S5 COMBISCREEN 4.6 x 50 mm (4 min.); Solvent A = 10% MeOH, 90% H2O, 0.1% TFA; Solvent B = 90% MeOH, 10% H2O, 0.1% TFA. LC/MS M+1 = 432.12. 1H NMR (400 MHz, CDCl3) δ ppm 0.90 (t, J=7.28 Hz, 9H), 1.17 - 1.62 (m, 21H), 4.45 (q, J=7.03 Hz, 2 H), and 6.81 (s, IH).
References:
Location in patent:Page/Page column 54-55

994-89-8
144 suppliers
$30.00/1g
861135-87-7
4 suppliers
$681.00/500g

126085-91-4
54 suppliers
$240.00/500mg
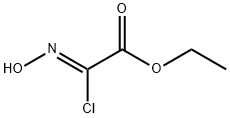
95080-93-6
1 suppliers
inquiry

994-89-8
144 suppliers
$30.00/1g

126085-91-4
54 suppliers
$240.00/500mg