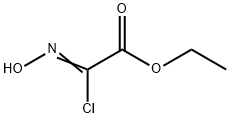
2-CHLORO-2-HYDROXYIMINOACETIC ACID ETHYL ESTER synthesis
- Product Name:2-CHLORO-2-HYDROXYIMINOACETIC ACID ETHYL ESTER
- CAS Number:14337-43-0
- Molecular formula:C4H6ClNO3
- Molecular Weight:151.55
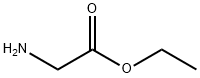
459-73-4

14337-43-0
General procedure for the synthesis of ethyl chlorooxime acetate from ethyl 2-aminoacetate: ethyl aminoacetate (5 g, 48.53 mmol) was dissolved in water (5 ml) to form a solution. To this solution, concentrated hydrochloric acid (8 ml) was slowly added at 0°C, followed by a mixture of sodium nitrite (NaNO2, 4.91 g, 145.6 mmol) and water (5 ml). The reaction mixture was stirred at room temperature for 1 hour. After completion of the reaction, the reaction mixture was extracted with ethyl acetate. The organic layers were combined and dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under vacuum to give ethyl chloroxime acetate (1.5 g, 20% yield) as a white solid.1H NMR (400 MHz, CDCl3): δ 1.37-1.41 (t, J = 7.2 Hz, 3H); 4.38-4.43 (q, J = 7.2 Hz, 2H). 9.59-9.61 (m, 1H).
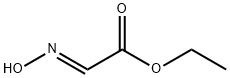
31767-15-4
0 suppliers
inquiry

14337-43-0
224 suppliers
$9.00/1g
Yield:14337-43-0 94%
Reaction Conditions:
with chlorine in dichloromethane
Steps:
1.2 EXAMPLE 1
(2) Into a solution of the above obtained ethyl 2-hydroxyiminoacetate (1.17 g) in dry methylene chloride (40 ml) is gradually introduced gaseous chlorine (3.6 g) at 0° C. with cooling over a cryogen, and the resulting mixture is stirred below 0° C. for 1 hour and then stirred at room temperature for 1 hour. The product is crystallized from benzene/n-hexane to give ethyl 2-chloro-2hydroxyiminoacetate as colorless needles melting at 75° to 80° C. Yield is 94%. IR (CHCl3): 1740 (ester), 3550 (hydroxy) cm-1
References:
Shionogi & Co., Ltd. US4190728, 1980, A

459-73-4
56 suppliers
inquiry

14337-43-0
224 suppliers
$9.00/1g
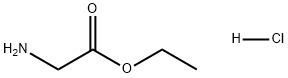
623-33-6
657 suppliers
$5.00/10g

14337-43-0
224 suppliers
$9.00/1g

623-33-6
657 suppliers
$5.00/10g

144-55-8
1411 suppliers
$10.00/25g

14337-43-0
224 suppliers
$9.00/1g
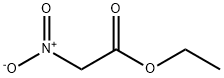
626-35-7
268 suppliers
$5.00/1g

14337-43-0
224 suppliers
$9.00/1g