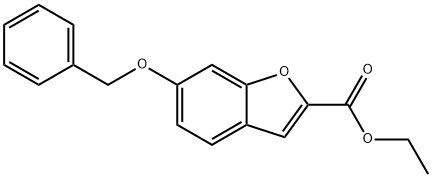
Ethyl 6-(Benzyloxy)benzofuran-2-carboxylate synthesis
- Product Name:Ethyl 6-(Benzyloxy)benzofuran-2-carboxylate
- CAS Number:110060-70-3
- Molecular formula:C18H16O4
- Molecular Weight:296.32
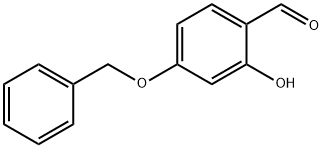
![Acetic acid, 2-[2-formyl-5-(phenylmethoxy)phenoxy]-, ethyl ester](/CAS/20210305/GIF/412300-65-3.gif)
412300-65-3
0 suppliers
inquiry

110060-70-3
18 suppliers
inquiry
Yield:110060-70-3 78%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in DMF (N,N-dimethyl-formamide) at 140; for 2 h;
Steps:
18.A
A solution of 4-benzyloxy-2-hydroxybenzaldehyde (7.90 g, 25.1 mmol) in acetone is treated with ethyl bromoacetate (5.79 g, 34.7 mmol) and potassium carbonate (7.98 g, 57.8 mmol). The mixture is stirred for 48 h at RT and filtered and concentrated, purified on column with 20% EtOAc/Hex to afford the intermediate ether (7.90 g, 87%). The intermediate is dissolved in DMF (40 mL) and treated with DBU (3.83 g, 25.1 mmol). The mixture is stirred at 140 °C for 2 h, cooled down, poured into water (500 mL) and extracted with EtOAc (2 x 100 mL). The organic layer is dried over Na2SO4, concentrated, purified on column chromatography (10-15% EtOAc/Hex) to afford the title compound (5.80 g, 78%). H-NMR (ppm, CDC13) USD : 7.53 (1 H, d, J=8. 8 Hz), 7.46 (1 H, s), 7.46 (1 H, s), 7. 44-7. 32 (4 H, m), 7.12 (1 H, d, J=2. 2 Hz), 7.02 (1 H, dd, J=2. 2,8. 4 Hz), 5.12 (2 H, s), 4.43 (2 H, q, J=7. 0 Hz), 1.42 (3 H, t, J=7. 0 Hz).
References:
WO2005/51938,2005,A1 Location in patent:Page/Page column 131-132
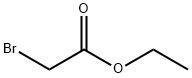
105-36-2
349 suppliers
$5.00/5G
52085-14-0
99 suppliers
$7.00/100mg

110060-70-3
18 suppliers
inquiry

52085-14-0
99 suppliers
$7.00/100mg

110060-70-3
18 suppliers
inquiry