Ethyl 6-chloro-2-(trifluoromethyl)nicotinate synthesis
- Product Name:Ethyl 6-chloro-2-(trifluoromethyl)nicotinate
- CAS Number:261635-82-9
- Molecular formula:C9H7ClF3NO2
- Molecular Weight:253.61
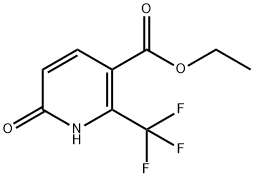
194673-13-7
26 suppliers
inquiry

261635-82-9
19 suppliers
inquiry
Yield:261635-82-9 68%
Reaction Conditions:
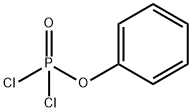
with O-phenyl phosphorodichloridate at 170; for 0.5 h;Microwave irradiation;
Steps:
Ethyl 6-chloro-2-(trifluoromethyl)pyridine-3-carboxylate (16)
A mixture of ethyl 6-hydroxy-2-(trifluoromethyl)pyridine-3-carboxylate (15) (890 mg, 3.78 mmol) and phenyl dichlorophosphate (0.85 ml_, 5.68 mmol) was heated under microwave irradiation for 30 min at 170°C. The reaction mixture was poured into ice, stirred for 20 min and diluted with ethyl acetate (50 ml_). The pH was adjusted to 10, by addition of a sat. aq. solution of sodium bicarbonate (50 ml_) and then the organic layer was separated, washed with water, dried over Na2S04 and concentrated under reduced pressure to give a brown oil which was purified by column chromatography (S1O2, 12 g, gradient elution 100% petrol to 50% EtOAc in petrol). The desired fractions were concentrated under reduced pressure to afford the title compound as a clear oil (650mg, 68%; 1 H NMR (500 MHz, Chloroform-d) d 8.09 (d, J = 8.3 Hz, 1 H), 7.60 (d, J = 8.2 Hz, 1 H), 4.44 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H); LCMS (4 minute method) product at Rt = 2.02 min and ES+ m/z 254.04, 256.02 [M+H]+ (Cl isotope)
References:
WO2019/166822,2019,A1 Location in patent:Page/Page column 70; 71
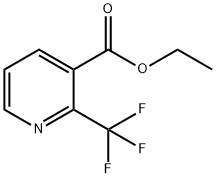
208517-35-5
132 suppliers
$45.00/50mg

261635-82-9
19 suppliers
inquiry
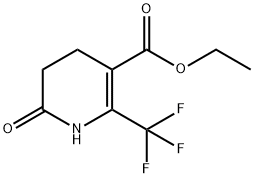
194673-12-6
61 suppliers
$7.00/100mg

261635-82-9
19 suppliers
inquiry
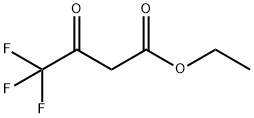
372-31-6
487 suppliers
$5.00/10g

261635-82-9
19 suppliers
inquiry